Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets
- PMID: 22685330
- PMCID: PMC3482316
- DOI: 10.1242/jcs.104943
Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets
Abstract
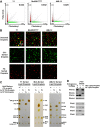
Perilipin family proteins (Plins) coat the surface of intracellular neutral lipid storage droplets in various cell types. Studies across diverse species demonstrate that Plins regulate lipid storage metabolism through recruitment of lipases and other regulatory proteins to lipid droplet surfaces. Mammalian genomes have distinct Plin gene members and additional protein forms derived from specific mRNA splice variants. However, it is not known if the different Plins have distinct functional properties. Using biochemical, cellular imaging and flow cytometric analyses, we now show that within individual cells of various types, the different Plin proteins preferentially sequester to separate pools of lipid storage droplets. By examining ectopically expressed GFP fusions and all endogenous Plin protein forms, we demonstrate that different Plins sequester to different types of lipid droplets that are composed of either triacylcerides or cholesterol esters. Furthermore, Plins with strong association preferences to triacylceride (or cholesterol ester) droplets can re-direct the relative intracellular triacylceride-cholesterol ester balance toward the targeted lipid. Our data suggest diversity of Plin function, alter previous assumptions about shared collective actions of the Plins, and indicate that each Plin can have separate and unique functions.
Figures






References
-
- Christian A. E., Haynes M. P., Phillips M. C., Rothblat G. H. (1997). Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38, 2264–2272 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

