Propagation of RML prions in mice expressing PrP devoid of GPI anchor leads to formation of a novel, stable prion strain
- PMID: 22685404
- PMCID: PMC3369955
- DOI: 10.1371/journal.ppat.1002746
Propagation of RML prions in mice expressing PrP devoid of GPI anchor leads to formation of a novel, stable prion strain
Abstract
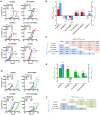
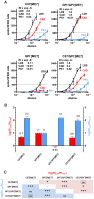
PrP(C), a host protein which in prion-infected animals is converted to PrP(Sc), is linked to the cell membrane by a GPI anchor. Mice expressing PrP(C) without GPI anchor (tgGPI⁻ mice), are susceptible to prion infection but accumulate anchorless PrP(Sc) extra-, rather than intracellularly. We investigated whether tgGPI⁻ mice could faithfully propagate prion strains despite the deviant structure and location of anchorless PrP(Sc). We found that RML and ME7, but not 22L prions propagated in tgGPI⁻ brain developed novel cell tropisms, as determined by the Cell Panel Assay (CPA). Surprisingly, the levels of proteinase K-resistant PrP(Sc) (PrP(res)) in RML- or ME7-infected tgGPI⁻ brain were 25-50 times higher than in wild-type brain. When returned to wild-type brain, ME7 prions recovered their original properties, however RML prions had given rise to a novel prion strain, designated SFL, which remained unchanged even after three passages in wild-type mice. Because both RML PrP(Sc) and SFL PrP(Sc) are stably propagated in wild-type mice we propose that the two conformations are separated by a high activation energy barrier which is abrogated in tgGPI⁻ mice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Endo T, Groth D, Prusiner SB, Kobata A. Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry. 1989;28:8380–8388. - PubMed
-
- Rudd PM, Wormald MR, Wing DR, Prusiner SB, Dwek RA. Prion glycoprotein: structure, dynamics, and roles for the sugars. Biochemistry. 2001;40:3759–3766. - PubMed
-
- Beringue V, Mallinson G, Kaisar M, Tayebi M, Sattar Z, et al. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain. 2003;126:2065–2073. - PubMed
-
- Somerville RA, Hamilton S, Fernie K. Transmissible spongiform encephalopathy strain, PrP genotype and brain region all affect the degree of glycosylation of PrPSc. J Gen Virol. 2005;86:241–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

