Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi
- PMID: 22685407
- PMCID: PMC3369953
- DOI: 10.1371/journal.ppat.1002750
Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi
Abstract
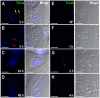
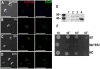
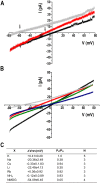
We report the identification, functional expression, purification, reconstitution and electrophysiological characterization of a novel cation channel (TcCat) from Trypanosoma cruzi, the etiologic agent of Chagas disease. This channel is potassium permeable and shows inward rectification in the presence of magnesium. Western blot analyses with specific antibodies indicated that the protein is expressed in the three main life cycle stages of the parasite. Surprisingly, the parasites have the unprecedented ability to rapidly change the localization of the channel when they are exposed to different environmental stresses. TcCat rapidly translocates to the tip of the flagellum when trypomastigotes are submitted to acidic pH, to the plasma membrane when epimastigotes are submitted to hyperosmotic stress, and to the cell surface when amastigotes are released to the extracellular medium. Pharmacological block of TcCat activity also resulted in alterations in the trypomastigotes ability to respond to hyperosmotic stress. We also demonstrate the feasibility of purifying and reconstituting a functional ion channel from T. cruzi after recombinant expression in bacteria. The peculiar characteristics of TcCat could be important for the development of specific inhibitors with therapeutic potential against trypanosomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bern C, Montgomery SP, Herwaldt BL, Rassi A, Jr, Marin-Neto JA, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007;298:2171–2181. - PubMed
-
- Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. Prognostic impact of Chagas disease in the United States. Am Heart J. 2009;157:22–29. - PubMed
-
- Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J Insect Physiol. 2001;47:739–747. - PubMed
-
- Van Der Heyden N, Docampo R. Intracellular pH in mammalian stages of Trypanosoma cruzi is K+-dependent and regulated by H+-ATPases. Mol Biochem Parasitol. 2000;105:237–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

