Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells
- PMID: 22685410
- PMCID: PMC3369929
- DOI: 10.1371/journal.ppat.1002762
Mobilization of HIV spread by diaphanous 2 dependent filopodia in infected dendritic cells
Abstract
Paramount to the success of persistent viral infection is the ability of viruses to navigate hostile environments en route to future targets. In response to such obstacles, many viruses have developed the ability of establishing actin rich-membrane bridges to aid in future infections. Herein through dynamic imaging of HIV infected dendritic cells, we have observed how viral high-jacking of the actin/membrane network facilitates one of the most efficient forms of HIV spread. Within infected DC, viral egress is coupled to viral filopodia formation, with more than 90% of filopodia bearing immature HIV on their tips at extensions of 10 to 20 µm. Live imaging showed HIV filopodia routinely pivoting at their base, and projecting HIV virions at µm.sec⁻¹ along repetitive arc trajectories. HIV filopodial dynamics lead to up to 800 DC to CD4 T cell contacts per hour, with selection of T cells culminating in multiple filopodia tethering and converging to envelope the CD4 T-cell membrane with budding HIV particles. Long viral filopodial formation was dependent on the formin diaphanous 2 (Diaph2), and not a dominant Arp2/3 filopodial pathway often associated with pathogenic actin polymerization. Manipulation of HIV Nef reduced HIV transfer 25-fold by reducing viral filopodia frequency, supporting the potency of DC HIV transfer was dependent on viral filopodia abundance. Thus our observations show HIV corrupts DC to CD4 T cell interactions by physically embedding at the leading edge contacts of long DC filopodial networks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
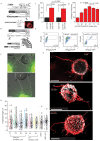
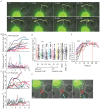
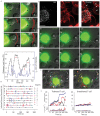

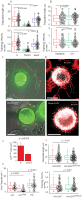

References
-
- Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. - PubMed
-
- Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. - PubMed
-
- Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

