RNA methylation by the MIS complex regulates a cell fate decision in yeast
- PMID: 22685417
- PMCID: PMC3369947
- DOI: 10.1371/journal.pgen.1002732
RNA methylation by the MIS complex regulates a cell fate decision in yeast
Abstract
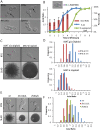
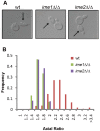
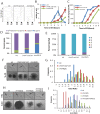
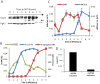
For the yeast Saccharomyces cerevisiae, nutrient limitation is a key developmental signal causing diploid cells to switch from yeast-form budding to either foraging pseudohyphal (PH) growth or meiosis and sporulation. Prolonged starvation leads to lineage restriction, such that cells exiting meiotic prophase are committed to complete sporulation even if nutrients are restored. Here, we have identified an earlier commitment point in the starvation program. After this point, cells, returned to nutrient-rich medium, entered a form of synchronous PH development that was morphologically and genetically indistinguishable from starvation-induced PH growth. We show that lineage restriction during this time was, in part, dependent on the mRNA methyltransferase activity of Ime4, which played separable roles in meiotic induction and suppression of the PH program. Normal levels of meiotic mRNA methylation required the catalytic domain of Ime4, as well as two meiotic proteins, Mum2 and Slz1, which interacted and co-immunoprecipitated with Ime4. This MIS complex (Mum2, Ime4, and Slz1) functioned in both starvation pathways. Together, our results support the notion that the yeast starvation response is an extended process that progressively restricts cell fate and reveal a broad role of post-transcriptional RNA methylation in these decisions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. - PubMed
-
- Dayani Y, Simchen G, Lichten M. Meiotic recombination intermediates are resolved with minimal crossover formation during return-to-growth, an analogue of the mitotic cell cycle. PLoS Genet. 2011;7:e1002083. doi: 10.1371/journal.pgen.1002083. - DOI - PMC - PubMed
-
- Kassir Y, Granot D, Simchen G. IME1, a positive regulator gene of meiosis in S. cerevisiae. Cell. 1988;52:853–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

