Semaphorin signaling in vertebrate neural circuit assembly
- PMID: 22685427
- PMCID: PMC3368236
- DOI: 10.3389/fnmol.2012.00071
Semaphorin signaling in vertebrate neural circuit assembly
Abstract
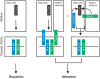
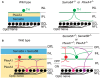
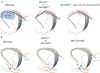
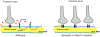
Neural circuit formation requires the coordination of many complex developmental processes. First, neurons project axons over long distances to find their final targets and then establish appropriate connectivity essential for the formation of neuronal circuitry. Growth cones, the leading edges of axons, navigate by interacting with a variety of attractive and repulsive axon guidance cues along their trajectories and at final target regions. In addition to guidance of axons, neuronal polarization, neuronal migration, and dendrite development must be precisely regulated during development to establish proper neural circuitry. Semaphorins consist of a large protein family, which includes secreted and cell surface proteins, and they play important roles in many steps of neural circuit formation. The major semaphorin receptors are plexins and neuropilins, however other receptors and co-receptors also mediate signaling by semaphorins. Upon semaphorin binding to their receptors, downstream signaling molecules transduce this event within cells to mediate further events, including alteration of microtubule and actin cytoskeletal dynamics. Here, I review recent studies on semaphorin signaling in vertebrate neural circuit assembly, with the goal of highlighting how this diverse family of cues and receptors imparts exquisite specificity to neural complex connectivity.
Keywords: axon guidance; neuropilin; plexin; semaphorin; synapse formation.
Figures

References
-
- Bagnard D., Lohrum M., Uziel D., Puschel A. W., Bolz J. (1998). Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 125, 5043–5053 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

