Prospective monitoring reveals dynamic levels of T cell immunity to Mycobacterium tuberculosis in HIV infected individuals
- PMID: 22685549
- PMCID: PMC3369919
- DOI: 10.1371/journal.pone.0037920
Prospective monitoring reveals dynamic levels of T cell immunity to Mycobacterium tuberculosis in HIV infected individuals
Abstract
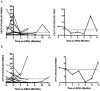
Monitoring of latent Mycobacterium tuberculosis infection may prevent disease. We tested an ESAT-6 and CFP-10-specific IFN-γ Elispot assay (RD1-Elispot) on 163 HIV-infected individuals living in a TB-endemic setting. An RD1-Elispot was performed every 3 months for a period of 3-21 months. 62% of RD1-Elispot negative individuals were positive by cultured Elispot. Fluctuations in T cell response were observed with rates of change ranging from -150 to +153 spot-forming cells (SFC)/200,000 PBMC in a 3-month period. To validate these responses we used an RD1-specific real time quantitative PCR assay for monokine-induced by IFN-γ (MIG) and IFN-γ inducible protein-10 (IP10) (MIG: r=0.6527, p=0.0114; IP-10: r=0.6967, p=0.0056; IP-10+MIG: r=0.7055, p=0.0048). During follow-up 30 individuals were placed on ARVs and 4 progressed to active TB. Fluctuations in SFC did not correlate with CD4 count, viral load, treatment initiation, or progression to active TB. The RD1-Elispot appears to have limited value in this setting.
Conflict of interest statement
Figures



References
-
- WHO (2010 guidelines under development) Policy statement on preventive therapy against TB in people living with HIV.
-
- Shafer RW, Edlin BR. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin Infect Dis. 1996;22:704. - PubMed
-
- Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis. 2009;13:46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

