Cancer Phenotype Diagnosis and Drug Efficacy within Japanese Health Care
- PMID: 22685658
- PMCID: PMC3364583
- DOI: 10.1155/2012/921901
Cancer Phenotype Diagnosis and Drug Efficacy within Japanese Health Care
Abstract
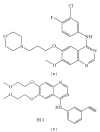
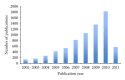
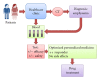
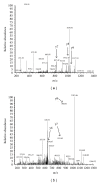
An overview on targeted personalized medicine is given describing the developments in Japan of lung cancer patients. These new targeted therapies with novel personalized medicine drugs require new implementations, in order to follow and monitor drug efficacy and outcome. Examples from IRESSA (Gefitinib) and TARCEVA (Erlotinib) treatments used in medication of lung cancer patients are presented. Lung cancer is one of the most common causes of cancer mortality in the world. The importance of both the quantification of disease progression, where diagnostic-related biomarkers are being implemented, in addition to the actual measurement of disease-specific mechanisms relating to pathway signalling activation of disease-progressive protein targets is summarised. An outline is also presented, describing changes and adaptations in Japan, meeting the rising costs and challenges. Today, urgent implementation of programs to address these needs has led to a rebuilding of the entire approach of medical evaluation and clinical care.
Figures

Similar articles
-
A clinician view and experience of proteomic studies in the light of lung cancer in Japanese healthcare.J Proteome Res. 2011 Jan 7;10(1):51-7. doi: 10.1021/pr100859b. J Proteome Res. 2011. PMID: 21141868
-
Drug localization in different lung cancer phenotypes by MALDI mass spectrometry imaging.J Proteomics. 2011 Jun 10;74(7):982-92. doi: 10.1016/j.jprot.2011.03.019. Epub 2011 Apr 1. J Proteomics. 2011. PMID: 21440690
-
Erlotinib: CP 358774, NSC 718781, OSI 774, R 1415.Drugs R D. 2003;4(4):243-8. doi: 10.2165/00126839-200304040-00006. Drugs R D. 2003. PMID: 12848590
-
Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC).Rev Recent Clin Trials. 2006 Jan;1(1):1-13. doi: 10.2174/157488706775246157. Rev Recent Clin Trials. 2006. PMID: 18393776 Review.
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
Cited by
-
Clinical initiatives linking Japanese and Swedish healthcare resources on cancer studies utilizing Biobank Repositories.Clin Transl Med. 2014 Nov 22;3(1):61. doi: 10.1186/s40169-014-0038-x. eCollection 2014 Dec. Clin Transl Med. 2014. PMID: 25635206 Free PMC article.
References
-
- Kaneko R. The society created by the longevity revolution–historical development and associated issues. Japanese Journal of Population. 2009;9(1):135–154.
-
- Kato H, Nishimura T, Hirano T, et al. A clinician view and experience of proteomic studies in the light of lung cancer in Japanese healthcare. Journal of Proteome Research. 2011;10(1):51–57. - PubMed
-
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (iressa survival evaluation in lung cancer) Lancet. 2005;366(9496):1527–1537. - PubMed
-
- Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in japanese patients with lung cancer: a cohort and nested case-control study. American Journal of Respiratory and Critical Care Medicine. 2008;177(12):1348–1357. - PubMed
-
- Davis JC, Furstenthal L, Desai AA, et al. The microeconomics of personalized medicine: today’s challenge and tomorrow’s promise. Nature Reviews Drug Discovery. 2009;8(4):279–286. - PubMed
LinkOut - more resources
Full Text Sources
