Glycal assembly by the in situ generation of glycosyl dithiocarbamates
- PMID: 22686424
- PMCID: PMC3412062
- DOI: 10.1021/ol301349w
Glycal assembly by the in situ generation of glycosyl dithiocarbamates
Abstract
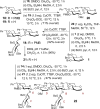
Glycal assembly offers an expedient entry into β-linked oligosaccharides, but epoxyglycal donors can be capricious in their reactivities. Treatment with Et(2)NH and CS(2) enables their in situ conversion into glycosyl dithiocarbamates, which can be activated by copper triflate for coupling with complex or sterically congested acceptors. The coupling efficiency can be further enhanced by in situ benzoylation, as illustrated in an 11-step synthesis of a branched hexasaccharide from glucals in 28% isolated yield and just four chromatographic purifications.
Figures


References
-
- Smoot JT, Demchenko AV. Adv. Carbohydr. Chem. Biochem. 2009;62:161. - PubMed
-
- Halcomb RL, Danishefsky SJ. J. Am. Chem. Soc. 1989;111:6661.
-
- Seeberger PH, Bilodeau MT, Danishefsky SJ. Aldrichimica Acta. 1997;30:75.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

