Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase
- PMID: 22686507
- PMCID: PMC3439328
- DOI: 10.1186/1475-2859-11-75
Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase
Abstract
Background: Microbial laccases are highly useful in textile effluent dye biodegradation. However, the bioavailability of cellularly expressed or purified laccases in continuous operations is usually limited by mass transfer impediment or enzyme regeneration difficulty. Therefore, this study develops a regenerable bacterial surface-displaying system for industrial synthetic dye decolorization, and evaluates its effects on independent and continuous operations.
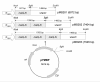
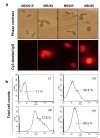
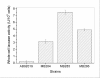
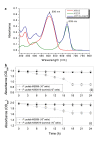
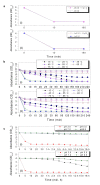
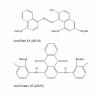
Results: A bacterial laccase (WlacD) was engineered onto the cell surface of the solvent-tolerant bacterium Pseudomonas putida to construct a whole-cell biocatalyst. Ice nucleation protein (InaQ) anchor was employed, and the ability of 1 to 3 tandemly aligned N-terminal repeats to direct WlacD display were compared. Immobilized WlacD was determined to be surface-displayed in functional form using Western blot analysis, immunofluorescence microscopy, flow cytometry, and whole-cell enzymatic activity assay. Engineered P. putida cells were then applied to decolorize the anthraquinone dye Acid Green (AG) 25 and diazo-dye Acid Red (AR) 18. The results showed that decolorization of both dyes is Cu(2+)- and mediator-independent, with an optimum temperature of 35°C and pH of 3.0, and can be stably performed across a temperature range of 15°C to 45°C. A high activity toward AG25 (1 g/l) with relative decolorization values of 91.2% (3 h) and 97.1% (18 h), as well as high activity to AR18 (1 g/l) by 80.5% (3 h) and 89.0% (18 h), was recorded. The engineered system exhibited a comparably high activity compared with those of separate dyes in a continuous three-round shake-flask decolorization of AG25/AR18 mixed dye (each 1 g/l). No significant decline in decolorization efficacy was noted during first two-rounds but reaction equilibriums were elongated, and the residual laccase activity eventually decreased to low levels. However, the decolorizing capacity of the system was easily retrieved via a subsequent 4-h cell culturing.
Conclusions: This study demonstrates, for the first time, the methodology by which the engineered P. putida with surface-immobilized laccase was successfully used as regenerable biocatalyst for biodegrading synthetic dyes, thereby opening new perspectives in the use of biocatalysis in industrial dye biotreatment.
Figures



References
-
- Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem. 2002;277:18849–18859. doi: 10.1074/jbc.M200827200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

