Glutathione complexed Fe-S centers
- PMID: 22687047
- PMCID: PMC3401418
- DOI: 10.1021/ja302186j
Glutathione complexed Fe-S centers
Abstract
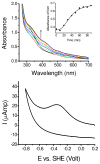
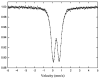
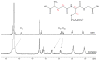
Glutathione (γ-glutamyl-cysteinyl-glycine, GSH) is a major thiol-containing peptide with cellular levels of up to 10 mM. (1) Several recent reports have demonstrated glutaredoxins (Grx) to form [Fe(2)S(2)] cluster-bridged dimers, where glutathione provides two exogenous thiol ligands, and have implicated such species in cellular iron sulfur cluster biosynthesis. We report the finding that glutathione alone can coordinate and stabilize an [Fe(2)S(2)] cluster under physiological conditions, with optical, redox, Mössbauer, and NMR characteristics that are consistent with a [Fe(2)S(2)](GS)(4) composition. The Fe-S assembly protein ISU catalyzes formation of [Fe(2)S(2)](GS)(4) from iron and sulfide ions in the presence of glutathione, and the [Fe(2)S(2)] core undergoes reversible exchange between apo ISU and free glutathione.
Figures
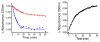
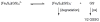
References
-
- Masip L, Veeravalli K, Georgiou G. Antioxid Redox Signal. 2006;8:753–62. - PubMed
-
- Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. Biochem Pharmacol. 2003;66:1499–503. - PubMed
-
- Meister A, Anderson ME. Annu Rev Biochem. 1983;52:711–60. - PubMed
-
- Sies H. Free Radic Biol Med. 1999;27:916–21. - PubMed
-
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Science. 1973;179:588–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

