A randomised controlled trial of a cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN): trial protocol
- PMID: 22687265
- PMCID: PMC3419080
- DOI: 10.1186/1471-2407-12-230
A randomised controlled trial of a cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN): trial protocol
Abstract
Background: This randomised controlled trial (RCT) aims to evaluate the effectiveness of a guided self-help cognitive behavioural intervention to alleviate problematic hot flushes (HF) and night sweats (NS) in men who are undergoing prostate cancer treatment. The trial and the self-help materials have been adapted from a previous RCT, which showed that a cognitive behavioural intervention reduced the self-reported problem-rating of hot flushes in women with menopausal symptoms, and in women undergoing breast cancer treatment. We hypothesize that guided self-help will be more effective than usual care in reducing HF/NS problem-rating at post treatment assessment.
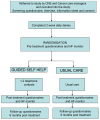
Methods/design: Seventy men who are undergoing treatment for prostate cancer and who have been experiencing more than ten HF/NS weekly for over a month are recruited into the trial from urology clinics in London. They are randomly allocated to either a four-week self-help cognitive behavioural therapy (CBT) treatment or to their usual care (control group). The treatment includes information and discussion about hot flushes and night sweats in the context of prostate cancer, monitoring and modifying precipitants, relaxation and paced respiration, stress management, cognitive therapy for unhelpful thoughts and beliefs, managing sleep and night sweats, and advice on maintaining these changes. Prior to randomisation, men attend a clinical interview, undergo 24-48-hour sternal skin conductance monitoring, and complete pre-treatment questionnaires (e.g., problem-rating and frequency of hot flushes and night sweats; quality of life; mood; hot flush beliefs and behaviours). Post-treatment measures (sternal skin conductance and the above questionnaires) are collected four-six weeks later, and again at a six-month follow-up.
Discussion: MANCAN is the first randomised controlled trial of cognitive behavioural therapy for HF/NS for men that measures both self-reported and physiologically indexed symptoms. The results will inform future clinical practice by evaluating an evidence-based, non-medical treatment, which can be delivered by trained health professionals.
Trial registration: UK Clinical Research Network UKCRN10904.
Figures
Similar articles
-
A randomised controlled trial of a brief cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN).Psychooncology. 2015 Sep;24(9):1159-66. doi: 10.1002/pon.3794. Epub 2015 Mar 9. Psychooncology. 2015. PMID: 25753889 Free PMC article. Clinical Trial.
-
A randomised controlled trial of a cognitive behavioural intervention for women who have menopausal symptoms following breast cancer treatment (MENOS 1): trial protocol.BMC Cancer. 2011 Jan 31;11:44. doi: 10.1186/1471-2407-11-44. BMC Cancer. 2011. PMID: 21281461 Free PMC article. Clinical Trial.
-
MENOS4 trial: a multicentre randomised controlled trial (RCT) of a breast care nurse delivered cognitive behavioural therapy (CBT) intervention to reduce the impact of hot flushes in women with breast cancer: Study Protocol.BMC Womens Health. 2018 May 8;18(1):63. doi: 10.1186/s12905-018-0550-z. BMC Womens Health. 2018. PMID: 29739384 Free PMC article. Clinical Trial.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
-
Mindfulness, cognitive behavioural and behaviour-based therapy for natural and treatment-induced menopausal symptoms: a systematic review and meta-analysis.BJOG. 2019 Feb;126(3):330-339. doi: 10.1111/1471-0528.15153. Epub 2018 Mar 15. BJOG. 2019. PMID: 29542222 Free PMC article.
Cited by
-
Cognitive Behavioral Therapy for Insomnia in Breast Cancer Survivors: A Review of the Literature.Front Psychol. 2016 Aug 3;7:1162. doi: 10.3389/fpsyg.2016.01162. eCollection 2016. Front Psychol. 2016. PMID: 27536265 Free PMC article. Review.
-
Acute Cancer Cognitive Therapy.Cogn Behav Pract. 2014 Nov;21(4):404-415. doi: 10.1016/j.cbpra.2014.03.003. Epub 2014 Mar 28. Cogn Behav Pract. 2014. PMID: 30555220 Free PMC article.
-
A randomised controlled trial of a brief cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN).Psychooncology. 2015 Sep;24(9):1159-66. doi: 10.1002/pon.3794. Epub 2015 Mar 9. Psychooncology. 2015. PMID: 25753889 Free PMC article. Clinical Trial.
-
Effectiveness of cognitive behavioural therapy on quality of life in patients with prostate cancer after androgen deprivation therapy: a protocol for systematic review and meta-analysis.BMJ Open. 2021 Nov 18;11(11):e049314. doi: 10.1136/bmjopen-2021-049314. BMJ Open. 2021. PMID: 34794990 Free PMC article. Clinical Trial.
-
Effects of occupational therapy on quality of life of patients with metastatic prostate cancer. A randomized controlled study.Saudi Med J. 2015 Aug;36(8):954-61. doi: 10.15537/smj.2015.8.11461. Saudi Med J. 2015. PMID: 26219446 Free PMC article. Clinical Trial.
References
-
- Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT. et al.Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. - DOI - PubMed
-
- Spetz AC, Hammer M. Incidence and management of hot flashes in prostate cancer. J Brit Menopause Society. 2002;1:57–62. - PubMed
-
- Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73(Suppl 5):28–35. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous