Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate
- PMID: 22687507
- PMCID: PMC3421567
- DOI: 10.1128/AAC.00231-12
Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate
Abstract
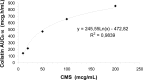
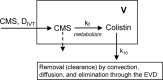
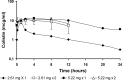
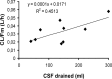
Intraventricular colistin, administered as colistin methanesulfonate (CMS), is the last resource for the treatment of central nervous system infections caused by panresistant Gram-negative bacteria. The doses and daily regimens vary considerably and are empirically chosen; the cerebrospinal fluid (CSF) pharmacokinetics of colistin after intraventricular administration of CMS has never been characterized. Nine patients (aged 18 to 73 years) were treated with intraventricular CMS (daily doses of 2.61 to 10.44 mg). Colistin concentrations were measured using a selective high-performance liquid chromatography (HPLC) assay. The population pharmacokinetics analysis was performed with the P-Pharm program. The pharmacokinetics of colistin could be best described by the one-compartment model. The estimated values (means ± standard deviations) of apparent CSF total clearance (CL/Fm, where Fm is the unknown fraction of CMS converted to colistin) and terminal half-life (t(1/2λ)) were 0.033 ± 0.014 liter/h and 7.8 ± 3.2 h, respectively, and the average time to the peak concentration was 3.7 ± 0.9 h. A positive correlation between CL/Fm and the amount of CSF drained (range 40 to 300 ml) was observed. When CMS was administered at doses of ≥5.22 mg/day, measured CSF concentrations of colistin were continuously above the MIC of 2 μg/ml, and measured values of trough concentration (C(trough)) ranged between 2.0 and 9.7 μg/ml. Microbiological cure was observed in 8/9 patients. Intraventricular administration of CMS at doses of ≥5.22 mg per day was appropriate in our patients, but since external CSF efflux is variable and can influence the clearance of colistin and its concentrations in CSF, the daily dose of 10 mg suggested by the Infectious Diseases Society of America may be more prudent.
Figures
References
-
- Bergen PJ, et al. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 61:636–642 - PubMed
-
- Cook AM, Mieure KD, Owen RD, Pesaturo AB, Hatton J. 2009. Intracerebroventricular administration of drugs. Pharmacotherapy 29:832–845 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

