Rapid redistribution of phosphatidylinositol-(4,5)-bisphosphate and septins during the Candida albicans response to caspofungin
- PMID: 22687514
- PMCID: PMC3421880
- DOI: 10.1128/AAC.00112-12
Rapid redistribution of phosphatidylinositol-(4,5)-bisphosphate and septins during the Candida albicans response to caspofungin
Abstract
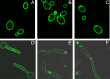
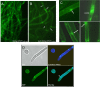
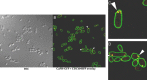
We previously showed that phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] and septin regulation play major roles in maintaining Candida albicans cell wall integrity in response to caspofungin and other stressors. Here, we establish a link between PI(4,5)P2 signaling and septin localization and demonstrate that rapid redistribution of PI(4,5)P2 and septins is part of the natural response of C. albicans to caspofungin. First, we studied caspofungin-hypersusceptible C. albicans irs4 and inp51 mutants, which have elevated PI(4,5)P2 levels due to loss of PI(4,5)P2-specific 5'-phosphatase activity. PI(4,5)P2 accumulated in discrete patches, rather than uniformly, along surfaces of mutants in yeast and filamentous morphologies, as visualized with a green fluorescent protein (GFP)-pleckstrin homology domain. The patches also contained chitin (calcofluor white staining) and cell wall protein Rbt5 (Rbt5-GFP). By transmission electron microscopy, patches corresponded to plasma membrane invaginations that incorporated cell wall material. Fluorescently tagged septins Cdc10 and Sep7 colocalized to these sites, consistent with well-described PI(4,5)P2-septin physical interactions. Based on expression patterns of cell wall damage response genes, irs4 and inp51 mutants were firmly positioned within a group of caspofungin-hypersusceptible, septin-regulatory protein kinase mutants. irs4 and inp51 were linked most closely to the gin4 mutant by expression profiling, PI(4,5)P2-septin-chitin redistribution and other phenotypes. Finally, sublethal 5-min exposure of wild-type C. albicans to caspofungin resulted in redistribution of PI(4,5)P2 and septins in a manner similar to those of irs4, inp51, and gin4 mutants. Taken together, our data suggest that the C. albicans Irs4-Inp51 5'-phosphatase complex and Gin4 function upstream of PI(4,5)P2 and septins in a pathway that helps govern responses to caspofungin.
Figures






Similar articles
-
Regulation of phosphatidylinositol-(4,5)-bisphosphate and active-Rho1p levels and distribution is crucial for correct spatio-temporal cytokinesis and echinocandin responses in Candida albicans.Antimicrob Agents Chemother. 2025 Jun 4;69(6):e0190024. doi: 10.1128/aac.01900-24. Epub 2025 May 5. Antimicrob Agents Chemother. 2025. PMID: 40323423 Free PMC article.
-
Highly Dynamic and Specific Phosphatidylinositol 4,5-Bisphosphate, Septin, and Cell Wall Integrity Pathway Responses Correlate with Caspofungin Activity against Candida albicans.Antimicrob Agents Chemother. 2016 May 23;60(6):3591-600. doi: 10.1128/AAC.02711-15. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27021331 Free PMC article.
-
The Candida albicans phosphatase Inp51p interacts with the EH domain protein Irs4p, regulates phosphatidylinositol-4,5-bisphosphate levels and influences hyphal formation, the cell integrity pathway and virulence.Microbiology (Reading). 2008 Nov;154(Pt 11):3296-3308. doi: 10.1099/mic.0.2008/018002-0. Microbiology (Reading). 2008. PMID: 18957583
-
Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: A distinct branch of PI3K signaling.Cell Signal. 2015 Sep;27(9):1789-98. doi: 10.1016/j.cellsig.2015.05.013. Epub 2015 May 27. Cell Signal. 2015. PMID: 26022180 Review.
-
Fungal pathogens are platforms for discovering novel and conserved septin properties.Curr Opin Microbiol. 2014 Aug;20:42-8. doi: 10.1016/j.mib.2014.04.004. Epub 2014 May 28. Curr Opin Microbiol. 2014. PMID: 24879478 Free PMC article. Review.
Cited by
-
Role of MCC/Eisosome in Fungal Lipid Homeostasis.Biomolecules. 2019 Jul 25;9(8):305. doi: 10.3390/biom9080305. Biomolecules. 2019. PMID: 31349700 Free PMC article. Review.
-
Plasma membrane organization promotes virulence of the human fungal pathogen Candida albicans.J Microbiol. 2016 Mar;54(3):178-91. doi: 10.1007/s12275-016-5621-y. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920878 Free PMC article. Review.
-
Beyond division and morphogenesis: Considering the emerging roles of septins in plasma membrane homeostasis and cell wall integrity in human fungal pathogens.PLoS Pathog. 2025 Jun 17;21(6):e1013226. doi: 10.1371/journal.ppat.1013226. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40526607 Free PMC article. No abstract available.
-
Regulation of phosphatidylinositol-(4,5)-bisphosphate and active-Rho1p levels and distribution is crucial for correct spatio-temporal cytokinesis and echinocandin responses in Candida albicans.Antimicrob Agents Chemother. 2025 Jun 4;69(6):e0190024. doi: 10.1128/aac.01900-24. Epub 2025 May 5. Antimicrob Agents Chemother. 2025. PMID: 40323423 Free PMC article.
-
A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis.Eukaryot Cell. 2012 Oct;11(10):1210-8. doi: 10.1128/EC.00216-12. Epub 2012 Aug 10. Eukaryot Cell. 2012. PMID: 22886998 Free PMC article.
References
-
- Badrane H, et al. 2005. Candida albicans IRS4 contributes to hyphal formation and virulence after the initial stages of disseminated candidiasis. Microbiology 151:2923–2931 - PubMed
-
- Badrane H, et al. 2008. The Candida albicans phosphatase Inp51p interacts with the EH domain protein Irs4p, regulates phosphatidylinositol-4,5-bisphosphate levels and influences hyphal formation, the cell integrity pathway and virulence. Microbiology 154:3296–3308 - PubMed
-
- Berman J, Sudbery PE. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918–930 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

