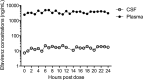
Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval
- PMID: 22687515
- PMCID: PMC3421893
- DOI: 10.1128/AAC.06311-11
Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval
Abstract
We determined the pharmacokinetics of efavirenz in plasma and cerebrospinal fluid (CSF) over a 24-h dosing interval in a patient who had undergone a lumbar drain because of cryptococcal meningitis. Drug concentrations were determined by high-performance liquid chromatography-tandem mass spectrometry in paired CSF (n = 24) and plasma (n = 25) samples. The median plasma efavirenz concentration was 3,718 ng/ml (range, 2,439 to 4,952), and the median CSF concentration was 16.3 ng/ml (range, 7.3 to 22.3). The CSF/plasma area-under-the-curve ratio was 0.0044 corresponding to a CSF penetration of 0.44% of plasma.
Figures
References
-
- Acosta E, et al. 2011. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA
-
- Antinori A, et al. 2005. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin. Infect. Dis. 41:1787–1793 - PubMed
-
- Cysique LA, Maruff P, Brew BJ. 2004. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J. Neurovirol. 10:350–357 - PubMed
-
- d'Arminio Monforte A, et al. 2004. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann. Neurol. 55:320–328 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical