Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle
- PMID: 22687611
- PMCID: PMC3547271
- DOI: 10.1113/jphysiol.2012.232777
Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle
Abstract
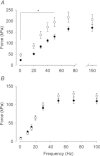
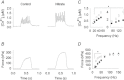
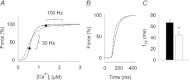
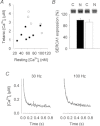
Dietary inorganic nitrate has profound effects on health and physiological responses to exercise. Here, we examined if nitrate, in doses readily achievable via a normal diet, could improve Ca(2+) handling and contractile function using fast- and slow-twitch skeletal muscles from C57bl/6 male mice given 1 mm sodium nitrate in water for 7 days. Age matched controls were provided water without added nitrate. In fast-twitch muscle fibres dissected from nitrate treated mice, myoplasmic free [Ca(2+)] was significantly greater than in Control fibres at stimulation frequencies from 20 to 150 Hz, which resulted in a major increase in contractile force at ≤ 50 Hz. At 100 Hz stimulation, the rate of force development was ∼35% faster in the nitrate group. These changes in nitrate treated mice were accompanied by increased expression of the Ca(2+) handling proteins calsequestrin 1 and the dihydropyridine receptor. No changes in force or calsequestrin 1 and dihydropyridine receptor expression were measured in slow-twitch muscles. In conclusion, these results show a striking effect of nitrate supplementation on intracellular Ca(2+) handling in fast-twitch muscle resulting in increased force production. A new mechanism is revealed by which nitrate can exert effects on muscle function with applications to performance and a potential therapeutic role in conditions with muscle weakness.
Figures

Similar articles
-
Piperine enhances contractile force in slow- and fast-twitch muscle.J Physiol. 2024 Jun;602(12):2807-2822. doi: 10.1113/JP285995. Epub 2024 May 19. J Physiol. 2024. PMID: 38762879
-
Beetroot Juice Increases Human Muscle Force without Changing Ca2+-Handling Proteins.Med Sci Sports Exerc. 2017 Oct;49(10):2016-2024. doi: 10.1249/MSS.0000000000001321. Med Sci Sports Exerc. 2017. PMID: 28509762
-
Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms.J Muscle Res Cell Motil. 1995 Feb;16(1):65-78. doi: 10.1007/BF00125311. J Muscle Res Cell Motil. 1995. PMID: 7751406
-
S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans.J Physiol. 2012 Mar 15;590(6):1443-63. doi: 10.1113/jphysiol.2011.224535. Epub 2012 Jan 16. J Physiol. 2012. PMID: 22250211 Free PMC article.
-
Fiber Type-Specific Effects of Dietary Nitrate.Exerc Sport Sci Rev. 2016 Apr;44(2):53-60. doi: 10.1249/JES.0000000000000074. Exerc Sport Sci Rev. 2016. PMID: 26829247 Review.
Cited by
-
Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature.Nutrients. 2023 Jan 28;15(3):660. doi: 10.3390/nu15030660. Nutrients. 2023. PMID: 36771366 Free PMC article. Review.
-
The Nitrate-Nitrite-Nitric Oxide Pathway on Healthy Ageing: A Review of Pre-clinical and Clinical Data on the Impact of Dietary Nitrate in the Elderly.Front Aging. 2021 Nov 17;2:778467. doi: 10.3389/fragi.2021.778467. eCollection 2021. Front Aging. 2021. PMID: 35821990 Free PMC article. Review.
-
Effects of Hydroxyurea on Skeletal Muscle Energetics and Function in a Mildly Anemic Mouse Model.Front Physiol. 2022 Jun 15;13:915640. doi: 10.3389/fphys.2022.915640. eCollection 2022. Front Physiol. 2022. PMID: 35784862 Free PMC article.
-
Mechanical isolation, and measurement of force and myoplasmic free [Ca2+] in fully intact single skeletal muscle fibers.Nat Protoc. 2017 Sep;12(9):1763-1776. doi: 10.1038/nprot.2017.056. Epub 2017 Aug 3. Nat Protoc. 2017. PMID: 28771237
-
A single-dose nitrate-producing dietary supplement affects cardiorespiratory endurance and muscular fitness in healthy men: A randomized controlled pilot trial.SAGE Open Med. 2021 Jul 29;9:20503121211036119. doi: 10.1177/20503121211036119. eCollection 2021. SAGE Open Med. 2021. PMID: 34377472 Free PMC article.
References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human Mol Genet. 2009;18:278–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

