Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling
- PMID: 22688333
- PMCID: PMC3425431
- DOI: 10.2337/db11-1462
Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling
Abstract
Insulin is secreted as discrete insulin secretory bursts at ~5-min intervals into the hepatic portal vein, these pulses being attenuated early in the development of type 1 and type 2 diabetes mellitus (T2DM). Intraportal insulin infusions (pulsatile, constant, or reproducing that in T2DM) indicated that the pattern of pulsatile insulin secretion delivered via the portal vein is important for hepatic insulin action and, therefore, presumably for hepatic insulin signaling. To test this, we examined hepatic insulin signaling in rat livers exposed to the same three patterns of portal vein insulin delivery by use of sequential liver biopsies in anesthetized rats. Intraportal delivery of insulin in a constant versus pulsatile pattern led to delayed and impaired activation of hepatic insulin receptor substrate (IRS)-1 and IRS-2 signaling, impaired activation of downstream insulin signaling effector molecules AKT and Foxo1, and decreased expression of glucokinase (Gck). We further established that hepatic Gck expression is decreased in the HIP rat model of T2DM, a defect that correlated with a progressive defect of pulsatile insulin secretion. We conclude that the physiological pulsatile pattern of insulin delivery is important in hepatic insulin signaling and glycemic control. Hepatic insulin resistance in diabetes is likely in part due to impaired pulsatile insulin secretion.
Figures





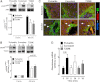

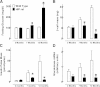
Comment in
-
Loss of pulsatile insulin secretion: a factor in the pathogenesis of type 2 diabetes?Diabetes. 2012 Sep;61(9):2228-9. doi: 10.2337/db12-0664. Diabetes. 2012. PMID: 22923651 Free PMC article. No abstract available.
References
-
- DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 1982;31:795–801 - PubMed
-
- Yki-Järvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med 1986;315:224–230 - PubMed
-
- Bock G, Chittilapilly E, Basu R, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 2007;56:1703–1711 - PubMed
-
- Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006;55:3536–3549 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

