The structural biology of enzymes involved in natural product glycosylation
- PMID: 22688446
- PMCID: PMC3627186
- DOI: 10.1039/c2np20039b
The structural biology of enzymes involved in natural product glycosylation
Abstract
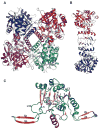
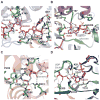
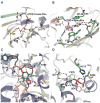
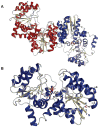
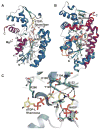
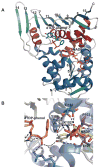
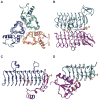
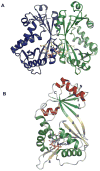
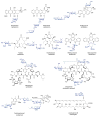
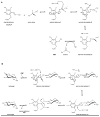
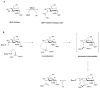
The glycosylation of microbial natural products often dramatically influences the biological and/or pharmacological activities of the parental metabolite. Over the past decade, crystal structures of several enzymes involved in the biosynthesis and attachment of novel sugars found appended to natural products have emerged. In many cases, these studies have paved the way to a better understanding of the corresponding enzyme mechanism of action and have served as a starting point for engineering variant enzymes to facilitate to production of differentially-glycosylated natural products. This review specifically summarizes the structural studies of bacterial enzymes involved in biosynthesis of novel sugar nucleotides.
Figures
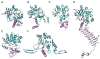








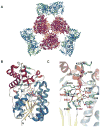



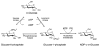

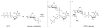




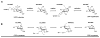





References
-
- Kilcoyne M, Joshi L. Cardiovasc Hematol Agents, Med Chem. 2007;5:186–197. - PubMed
-
- Kren V, Martinkova L. Curr Med Chem. 2001;8:1303–1328. - PubMed
-
- Kren V, Rezanka T. FEMS Microbiol Rev. 2008;32:858–889. - PubMed
-
- Wrodnigg TM, Sprenger FK. Mini Rev Med Chem. 2004;4:437–459. - PubMed
-
- Thorson JS, Hosted TJ, Jiang JQ, Biggins JB, Ahlert J. Curr Org Chem. 2001;5:139–167.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

