Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system
- PMID: 22688810
- PMCID: PMC3372250
- DOI: 10.1128/MMBR.05018-11
Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system
Abstract
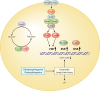
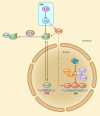
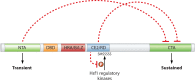
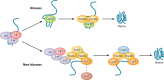
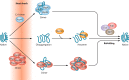
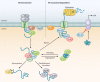
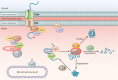
The eukaryotic heat shock response is an ancient and highly conserved transcriptional program that results in the immediate synthesis of a battery of cytoprotective genes in the presence of thermal and other environmental stresses. Many of these genes encode molecular chaperones, powerful protein remodelers with the capacity to shield, fold, or unfold substrates in a context-dependent manner. The budding yeast Saccharomyces cerevisiae continues to be an invaluable model for driving the discovery of regulatory features of this fundamental stress response. In addition, budding yeast has been an outstanding model system to elucidate the cell biology of protein chaperones and their organization into functional networks. In this review, we evaluate our understanding of the multifaceted response to heat shock. In addition, the chaperone complement of the cytosol is compared to those of mitochondria and the endoplasmic reticulum, organelles with their own unique protein homeostasis milieus. Finally, we examine recent advances in the understanding of the roles of protein chaperones and the heat shock response in pathogenic fungi, which is being accelerated by the wealth of information gained for budding yeast.
Figures





References
-
- Abbas-Terki T, Donze O, Picard D. 2000. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 467:111–116 - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. 2003. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299:1751–1753 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

