Cell biology of cnidarian-dinoflagellate symbiosis
- PMID: 22688813
- PMCID: PMC3372257
- DOI: 10.1128/MMBR.05014-11
Cell biology of cnidarian-dinoflagellate symbiosis
Abstract
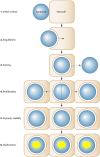
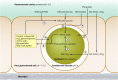
The symbiosis between cnidarians (e.g., corals or sea anemones) and intracellular dinoflagellate algae of the genus Symbiodinium is of immense ecological importance. In particular, this symbiosis promotes the growth and survival of reef corals in nutrient-poor tropical waters; indeed, coral reefs could not exist without this symbiosis. However, our fundamental understanding of the cnidarian-dinoflagellate symbiosis and of its links to coral calcification remains poor. Here we review what we currently know about the cell biology of cnidarian-dinoflagellate symbiosis. In doing so, we aim to refocus attention on fundamental cellular aspects that have been somewhat neglected since the early to mid-1980s, when a more ecological approach began to dominate. We review the four major processes that we believe underlie the various phases of establishment and persistence in the cnidarian/coral-dinoflagellate symbiosis: (i) recognition and phagocytosis, (ii) regulation of host-symbiont biomass, (iii) metabolic exchange and nutrient trafficking, and (iv) calcification. Where appropriate, we draw upon examples from a range of cnidarian-alga symbioses, including the symbiosis between green Hydra and its intracellular chlorophyte symbiont, which has considerable potential to inform our understanding of the cnidarian-dinoflagellate symbiosis. Ultimately, we provide a comprehensive overview of the history of the field, its current status, and where it should be going in the future.
Figures







References
-
- Alberts B, et al. 2008. Molecular biology of the cell, 4th ed Garland Science, New York, NY
-
- Al-Horani F, Al-Moghrabi SM, de Beer D. 2003. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: active internal carbon cycle. J. Exp. Mar. Biol. Ecol. 288:1–15
-
- Allemand D, Furla P, Bénazet-Tambutté S. 1998. Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can. J. Bot. 76:925–941
-
- Allemand D, Tambutté E, Girard JP, Jaubert J. 1998. Organic matrix synthesis in the scleractinian coral Stylophora pistillata: role in biomineralization and potential target of the organotin tributyltin. J. Exp. Biol. 201:2001–2009 - PubMed
-
- Allemand D, et al. 2004. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C. R. Palevol. 3:453–467
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

