Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria
- PMID: 22688814
- PMCID: PMC3372255
- DOI: 10.1128/MMBR.05017-11
Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria
Abstract
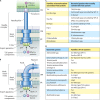
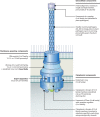
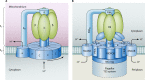
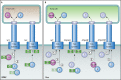
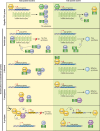
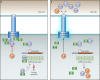
Flagellar and translocation-associated type III secretion (T3S) systems are present in most gram-negative plant- and animal-pathogenic bacteria and are often essential for bacterial motility or pathogenicity. The architectures of the complex membrane-spanning secretion apparatuses of both systems are similar, but they are associated with different extracellular appendages, including the flagellar hook and filament or the needle/pilus structures of translocation-associated T3S systems. The needle/pilus is connected to a bacterial translocon that is inserted into the host plasma membrane and mediates the transkingdom transport of bacterial effector proteins into eukaryotic cells. During the last 3 to 5 years, significant progress has been made in the characterization of membrane-associated core components and extracellular structures of T3S systems. Furthermore, transcriptional and posttranscriptional regulators that control T3S gene expression and substrate specificity have been described. Given the architecture of the T3S system, it is assumed that extracellular components of the secretion apparatus are secreted prior to effector proteins, suggesting that there is a hierarchy in T3S. The aim of this review is to summarize our current knowledge of T3S system components and associated control proteins from both plant- and animal-pathogenic bacteria.
Figures




References
-
- Abe A, et al. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162–1175 - PubMed
-
- Agrain C, et al. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 56:54–67 - PubMed
-
- Aili M, et al. 2008. Regulation of Yersinia Yop-effector delivery by translocated YopE. Int. J. Med. Microbiol. 298:183–192 - PubMed
-
- Akeda Y, Galan JE. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437:911–915 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

