Biochemistry and evolution of anaerobic energy metabolism in eukaryotes
- PMID: 22688819
- PMCID: PMC3372258
- DOI: 10.1128/MMBR.05024-11
Biochemistry and evolution of anaerobic energy metabolism in eukaryotes
Abstract
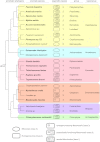
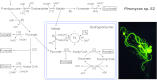
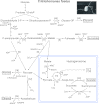
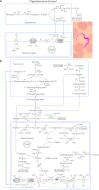
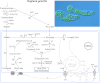
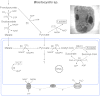
Major insights into the phylogenetic distribution, biochemistry, and evolutionary significance of organelles involved in ATP synthesis (energy metabolism) in eukaryotes that thrive in anaerobic environments for all or part of their life cycles have accrued in recent years. All known eukaryotic groups possess an organelle of mitochondrial origin, mapping the origin of mitochondria to the eukaryotic common ancestor, and genome sequence data are rapidly accumulating for eukaryotes that possess anaerobic mitochondria, hydrogenosomes, or mitosomes. Here we review the available biochemical data on the enzymes and pathways that eukaryotes use in anaerobic energy metabolism and summarize the metabolic end products that they generate in their anaerobic habitats, focusing on the biochemical roles that their mitochondria play in anaerobic ATP synthesis. We present metabolic maps of compartmentalized energy metabolism for 16 well-studied species. There are currently no enzymes of core anaerobic energy metabolism that are specific to any of the six eukaryotic supergroup lineages; genes present in one supergroup are also found in at least one other supergroup. The gene distribution across lineages thus reflects the presence of anaerobic energy metabolism in the eukaryote common ancestor and differential loss during the specialization of some lineages to oxic niches, just as oxphos capabilities have been differentially lost in specialization to anoxic niches and the parasitic life-style. Some facultative anaerobes have retained both aerobic and anaerobic pathways. Diversified eukaryotic lineages have retained the same enzymes of anaerobic ATP synthesis, in line with geochemical data indicating low environmental oxygen levels while eukaryotes arose and diversified.
Figures













References
-
- Abe T, Hoshino T, Nakamura A, Takaya N. 2007. Anaerobic elemental sulfur reduction by fungus Fusarium oxysporum. Biosci. Biotechnol. Biochem. 71:2402–2407 - PubMed
-
- Abhishek A, Bavishi A, Choudhary M. 2011. Bacterial genome chimaerism and the origin of mitochondria. Can. J. Microbiol. 57:49–61 - PubMed
-
- Adl SM, et al. 2005. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 52:399–451 - PubMed
-
- Aguilera P, Barry T, Tovar J. 2008. Entamoeba histolytica mitosomes: organelles in search of a function. Exp. Parasitol. 118:10–16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

