Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody
- PMID: 22689074
- PMCID: PMC3517000
- DOI: 10.1002/adma.201200776
Multivalent porous silicon nanoparticles enhance the immune activation potency of agonistic CD40 antibody
Abstract
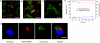
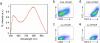
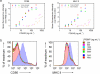
One of the fundamental paradigms in the use of nanoparticles to treat disease is to evade or suppress the immune system in order to minimize systemic side effects and deliver sufficient nanoparticle quantities to the intended tissues. However, the immune system is the body's most important and effective defense against diseases. It protects the host by identifying and eliminating foreign pathogens as well as self-malignancies. Here we report a nanoparticle engineered to work with the immune system, enhancing the intended activation of antigen presenting cells (APCs). We show that luminescent porous silicon nanoparticles (LPSiNPs), each containing multiple copies of an agonistic antibody (FGK45) to the APC receptor CD40, greatly enhance activation of B cells. The cellular response to the nanoparticle-based stimulators is equivalent to a 30-40 fold larger concentration of free FGK45. The intrinsic near-infrared photoluminescence of LPSiNPs is used to monitor degradation and track the nanoparticles inside APCs.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Tasciotti E, Liu XW, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MMC, Decuzzi P, Tour JM, Robertson F, Ferrari M. Nat. Nanotechnol. 2008;3:151. - PubMed
-
- Salonen J, Laitinen L, Kaukonen AM, Tuura J, Bjorkqvist M, Heikkila T, Vaha-Heikkila K, Hirvonen J, Lehto VP. J. Control. Release. 2005;108:362. - PubMed
-
- Low SP, Voelcker NH, Canham LT, Willams KA. Biomaterials. 2009;30:2873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

