A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer
- PMID: 22689129
- PMCID: PMC3549301
- DOI: 10.1038/pcan.2012.20
A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer
Abstract
Background: Pomegranate juice has been associated with PSA doubling time (PSADT) elongation in a single-arm phase II trial. This study assesses biological activity of two doses of pomegranate extract (POMx) in men with recurrent prostate cancer, using changes in PSADT as the primary outcome.
Methods: This randomized, multi-center, double-blind phase II, dose-exploring trial randomized men with a rising PSA and without metastases to receive 1 or 3 g of POMx, stratified by baseline PSADT and Gleason score. Patients (104) were enrolled and treated for up to 18 months. The intent-to-treat (ITT) population was 96% white, with median age 74.5 years and median Gleason score 7. This study was designed to detect a 6-month on-study increase in PSADT from baseline in each arm.
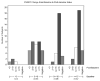
Results: Overall, median PSADT in the ITT population lengthened from 11.9 months at baseline to 18.5 months after treatment (P < 0.001). PSADT lengthened in the low-dose group from 11.9 to 18.8 months and 12.2 to 17.5 months in the high-dose group, with no significant difference between dose groups (P = 0.554). PSADT increases >100% of baseline were observed in 43% of patients. Declining PSA levels were observed in 13 patients (13%). In all, 42% of patients discontinued treatment before meeting the protocol-definition of PSA progression, or 18 months, primarily due to a rising PSA. No significant changes occurred in testosterone. Although no clinically significant toxicities were seen, diarrhea was seen in 1.9% and 13.5% of patients in the 1- and 3-g dose groups, respectively.
Conclusions: POMx treatment was associated with ≥ 6 month increases in PSADT in both treatment arms without adverse effects. The significance of this on-study slowing of PSADT remains unclear, reinforcing the need for placebo-controlled studies in this patient population.
Trial registration: ClinicalTrials.gov NCT01220817.
Conflict of interest statement
The corresponding author, Michael Carducci, received $1500 in 2007 from POM Wonderful, LLC, for participating in a discussion of future trials. Harley Liker and Patricia Wozniak are consultants to POM Wonderful, LLC, and Brad Gillespie was, until 2011, the vice president of POM Wonderful. The remaining authors declare no conflict of interest.
Figures

References
-
- Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170:1390–1395. - PubMed
-
- Pound CR, Christens-Barry OW, Gurganus RT, Partin AW, Walsh PC. Digital rectal examination and imaging studies are unnecessary in men with undetectable prostate specific antigen following radical prostatectomy. J Urol. 1999;162:1337–1340. - PubMed
-
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. - PubMed
-
- Antonarakis ES, Chen Y, Elsamanoudi SI, Brassell SA, Da Rocha MV, Eisenberger MA, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2010;108:378–385. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

