Slits affect the timely migration of neural crest cells via Robo receptor
- PMID: 22689303
- PMCID: PMC3632352
- DOI: 10.1002/dvdy.23817
Slits affect the timely migration of neural crest cells via Robo receptor
Abstract
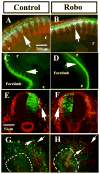
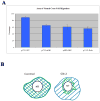
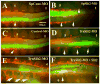
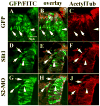
Background: Neural crest cells emerge by delamination from the dorsal neural tube and give rise to various components of the peripheral nervous system in vertebrate embryos. These cells change from non-motile into highly motile cells migrating to distant areas before further differentiation. Mechanisms controlling delamination and subsequent migration of neural crest cells are not fully understood. Slit2, a chemorepellant for axonal guidance that repels and stimulates motility of trunk neural crest cells away from the gut has recently been suggested to be a tumor suppressor molecule. The goal of this study was to further investigate the role of Slit2 in trunk neural crest cell migration by constitutive expression in neural crest cells.
Results: We found that Slit gain-of-function significantly impaired neural crest cell migration while Slit loss-of-function favored migration. In addition, we observed that the distribution of key cytoskeletal markers was disrupted in both gain and loss of function instances.
Conclusions: These findings suggest that Slit molecules might be involved in the processes that allow neural crest cells to begin migrating and transitioning to a mesenchymal type.
Copyright © 2012 Wiley Periodicals, Inc.
Figures







Similar articles
-
Screen for Slit/Robo signaling in trunk neural cells reveals new players.Gene Expr Patterns. 2018 Jun;28:22-33. doi: 10.1016/j.gep.2018.01.002. Epub 2018 Feb 7. Gene Expr Patterns. 2018. PMID: 29427758 Free PMC article.
-
Slit molecules prevent entrance of trunk neural crest cells in developing gut.Int J Dev Neurosci. 2015 Apr;41:8-16. doi: 10.1016/j.ijdevneu.2014.12.003. Epub 2014 Dec 6. Int J Dev Neurosci. 2015. PMID: 25490618 Free PMC article.
-
Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk.Dev Biol. 2005 Jun 15;282(2):411-21. doi: 10.1016/j.ydbio.2005.03.021. Dev Biol. 2005. PMID: 15950606
-
The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system.Reproduction. 2010 Apr;139(4):697-704. doi: 10.1530/REP-10-0017. Epub 2010 Jan 25. Reproduction. 2010. PMID: 20100881 Free PMC article. Review.
-
Molecular control of neural crest formation, migration and differentiation.Curr Opin Cell Biol. 2000 Dec;12(6):719-24. doi: 10.1016/s0955-0674(00)00158-7. Curr Opin Cell Biol. 2000. PMID: 11063938 Review.
Cited by
-
Maximal Expression of the Evolutionarily Conserved Slit2 Gene Promoter Requires Sp1.Cell Mol Neurobiol. 2016 Aug;36(6):955-964. doi: 10.1007/s10571-015-0281-8. Epub 2015 Oct 11. Cell Mol Neurobiol. 2016. PMID: 26456684 Free PMC article.
-
Slit and Semaphorin signaling governed by Islet transcription factors positions motor neuron somata within the neural tube.Exp Neurol. 2015 Jul;269:17-27. doi: 10.1016/j.expneurol.2015.03.024. Epub 2015 Apr 3. Exp Neurol. 2015. PMID: 25843547 Free PMC article.
-
Screen for Slit/Robo signaling in trunk neural cells reveals new players.Gene Expr Patterns. 2018 Jun;28:22-33. doi: 10.1016/j.gep.2018.01.002. Epub 2018 Feb 7. Gene Expr Patterns. 2018. PMID: 29427758 Free PMC article.
-
Slit molecules prevent entrance of trunk neural crest cells in developing gut.Int J Dev Neurosci. 2015 Apr;41:8-16. doi: 10.1016/j.ijdevneu.2014.12.003. Epub 2014 Dec 6. Int J Dev Neurosci. 2015. PMID: 25490618 Free PMC article.
-
Molecular Events Controlling Cessation of Trunk Neural Crest Migration and Onset of Differentiation.Front Cell Dev Biol. 2020 Apr 2;8:199. doi: 10.3389/fcell.2020.00199. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32318567 Free PMC article.
References
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. - PubMed
-
- Brown A, Li Y, Slaughter T, Black MM. Composite microtubules of the axon - Quantitative-analysis of tyrosinated and acetylated tubuling along individual axonal microtubules. Journal of Cell Science. 1993;104:339–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

