The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria
- PMID: 22689574
- PMCID: PMC3397900
- DOI: 10.1074/jbc.C112.378364
The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria
Abstract
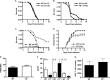
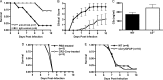
Cerebral malaria (CM) is the most severe manifestation of clinical malaria syndromes and has a high fatality rate especially in the developing world. Recent studies demonstrated that C5(-/-) mice are resistant to experimental CM (ECM) and that protection was due to the inability to form the membrane attack complex. Unexpectedly, we observed that C4(-/-) and factor B(-/-) mice were fully susceptible to disease, indicating that activation of the classical or alternative pathways is not required for ECM. C3(-/-) mice were also susceptible to ECM, indicating that the canonical C5 convertases are not required for ECM development and progression. Abrogation of ECM by treatment with anti-C9 antibody and detection of C5a in serum of C3(-/-) mice confirmed that C5 activation occurs in ECM independent of C5 convertases. Our data indicate that activation of C5 in ECM likely occurs via coagulation enzymes of the extrinsic protease pathway.
Figures
Similar articles
-
Protection of the classical and alternative complement pathway C3 convertases, stabilized by nephritic factors, from decay by the human C3b receptor.Eur J Immunol. 1984 Dec;14(12):1111-4. doi: 10.1002/eji.1830141209. Eur J Immunol. 1984. PMID: 6240408
-
Cutting edge: the membrane attack complex of complement is required for the development of murine experimental cerebral malaria.J Immunol. 2011 Jun 15;186(12):6657-60. doi: 10.4049/jimmunol.1100603. Epub 2011 May 13. J Immunol. 2011. PMID: 21572031 Free PMC article.
-
Molecular basis of complement resistance of human melanoma cells expressing the C3-cleaving membrane protease p65.Cancer Res. 1993 Feb 1;53(3):592-9. Cancer Res. 1993. PMID: 8425193
-
The proteolytic activation systems of complement.Annu Rev Biochem. 1981;50:433-64. doi: 10.1146/annurev.bi.50.070181.002245. Annu Rev Biochem. 1981. PMID: 7023363 Review. No abstract available.
-
Pathways of complement activation in membranoproliferative glomerulonephritis and allograft rejection.Transplant Proc. 1977 Mar;9(1):729-39. Transplant Proc. 1977. PMID: 325806 Review.
Cited by
-
Lack of long-lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum.Infect Immun. 2014 Jul;82(7):2688-96. doi: 10.1128/IAI.00055-14. Epub 2014 Apr 7. Infect Immun. 2014. PMID: 24711570 Free PMC article.
-
Intrathecal activation as a typical immune response within the central nervous system in angiostrongyliasis.Am J Trop Med Hyg. 2013 Feb;88(2):230-5. doi: 10.4269/ajtmh.12-0151. Am J Trop Med Hyg. 2013. PMID: 23390222 Free PMC article. Review.
-
The Complement System Is Essential for Arteriogenesis by Enhancing Sterile Inflammation as a Relevant Step in Collateral Artery Growth.Cells. 2024 Aug 23;13(17):1405. doi: 10.3390/cells13171405. Cells. 2024. PMID: 39272977 Free PMC article.
-
Mouse NOD/Shi and NSY/Hos strains infected with Plasmodium berghei ANKA are models for experimental cerebral malaria.Exp Anim. 2025 Jan 10;74(1):31-38. doi: 10.1538/expanim.24-0023. Epub 2024 Jul 26. Exp Anim. 2025. PMID: 39069480 Free PMC article.
-
Protein therapeutics and their lessons: Expect the unexpected when inhibiting the multi-protein cascade of the complement system.Immunol Rev. 2023 Jan;313(1):376-401. doi: 10.1111/imr.13164. Epub 2022 Nov 18. Immunol Rev. 2023. PMID: 36398537 Free PMC article. Review.
References
-
- Haldar K., Murphy S. C., Milner D. A., Taylor T. E. (2007) Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu. Rev. Pathol. 2, 217–249 - PubMed
-
- Grau G. E., Craig A. G. (2012) Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 7, 291–302 - PubMed
-
- Silver K. L., Higgins S. J., McDonald C. R., Kain K. C. (2010) Complement-driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol. 12, 1036–1045 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

