Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions
- PMID: 22689576
- PMCID: PMC3411067
- DOI: 10.1074/jbc.M112.374629
Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions
Abstract
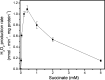
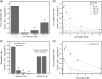
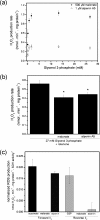
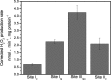
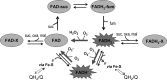
Respiratory complex II oxidizes succinate to fumarate as part of the Krebs cycle and reduces ubiquinone in the electron transport chain. Previous experimental evidence suggested that complex II is not a significant contributor to the production of reactive oxygen species (ROS) in isolated mitochondria or intact cells unless mutated. However, we find that when complex I and complex III are inhibited and succinate concentration is low, complex II in rat skeletal muscle mitochondria can generate superoxide or H(2)O(2) at high rates. These rates approach or exceed the maximum rates achieved by complex I or complex III. Complex II generates these ROS in both the forward reaction, with electrons supplied by succinate, and the reverse reaction, with electrons supplied from the reduced ubiquinone pool. ROS production in the reverse reaction is prevented by inhibition of complex II at either the ubiquinone-binding site (by atpenin A5) or the flavin (by malonate), whereas ROS production in the forward reaction is prevented by malonate but not by atpenin A5, showing that the ROS from complex II arises only from the flavin site (site II(F)). We propose a mechanism for ROS production by complex II that relies upon the occupancy of the substrate oxidation site and the reduction state of the enzyme. We suggest that complex II may be an important contributor to physiological and pathological ROS production.
Figures


References
-
- Bunik V. I., Sievers C. (2002) Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur. J. Biochem. 269, 5004–5015 - PubMed
-
- Adam-Vizi V. (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid. Redox. Signal. 7, 1140–1149 - PubMed
-
- Starkov A. A., Fiskum G. (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 86, 1101–1107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

