HIF-2α suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells
- PMID: 22689594
- PMCID: PMC3584519
- DOI: 10.1002/stem.1142
HIF-2α suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells
Abstract
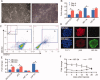
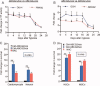
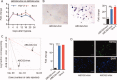
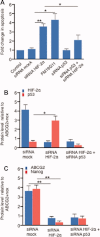
Human embryonic stem cells (hESCs) have been reported to exert cytoprotective activity in the area of tissue injury. However, hypoxia/oxidative stress prevailing in the area of injury could activate p53, leading to death and differentiation of hESCs. Here we report that when exposed to hypoxia/oxidative stress, a small fraction of hESCs, namely the SSEA3+/ABCG2+ fraction undergoes a transient state of reprogramming to a low p53 and high hypoxia inducible factor (HIF)-2α state of transcriptional activity. This state can be sustained for a period of 2 weeks and is associated with enhanced transcriptional activity of Oct-4 and Nanog, concomitant with high teratomagenic potential. Conditioned medium obtained from the post-hypoxia SSEA3+/ABCG2+ hESCs showed cytoprotection both in vitro and in vivo. We termed this phenotype as the "enhanced stemness" state. We then demonstrated that the underlying molecular mechanism of this transient phenotype of enhanced stemness involved high Bcl-2, fibroblast growth factor (FGF)-2, and MDM2 expression and an altered state of the p53/MDM2 oscillation system. Specific silencing of HIF-2α and p53 resisted the reprogramming of SSEA3+/ABCG2+ to the enhanced stemness phenotype. Thus, our studies have uncovered a unique transient reprogramming activity in hESCs, the enhanced stemness reprogramming where a highly cytoprotective and undifferentiated state is achieved by transiently suppressing p53 activity. We suggest that this transient reprogramming is a form of stem cell altruism that benefits the surrounding tissues during the process of tissue regeneration.
Copyright © 2012 AlphaMed Press.
Figures

Comment in
-
HIF-2α - a mediator of stem cell altruism?Stem Cell Res Ther. 2012 Dec 18;3(6):52. doi: 10.1186/scrt143. Stem Cell Res Ther. 2012. PMID: 23253294 Free PMC article.
Similar articles
-
HIF-2α - a mediator of stem cell altruism?Stem Cell Res Ther. 2012 Dec 18;3(6):52. doi: 10.1186/scrt143. Stem Cell Res Ther. 2012. PMID: 23253294 Free PMC article.
-
HIF-2α regulates NANOG expression in human embryonic stem cells following hypoxia and reoxygenation through the interaction with an Oct-Sox cis regulatory element.PLoS One. 2014 Oct 1;9(10):e108309. doi: 10.1371/journal.pone.0108309. eCollection 2014. PLoS One. 2014. PMID: 25271810 Free PMC article.
-
Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells.Circ Res. 2008 May 9;102(9):1075-81. doi: 10.1161/CIRCRESAHA.107.161729. Epub 2008 Mar 20. Circ Res. 2008. PMID: 18356544
-
Hypoxia, pseudohypoxia and cellular differentiation.Exp Cell Res. 2017 Jul 15;356(2):192-196. doi: 10.1016/j.yexcr.2017.03.007. Epub 2017 Mar 8. Exp Cell Res. 2017. PMID: 28284840 Review.
-
p53: emerging roles in stem cells, development and beyond.Development. 2018 Apr 13;145(8):dev158360. doi: 10.1242/dev.158360. Development. 2018. PMID: 29654218 Review.
Cited by
-
The Interplay Between Tumor Suppressor p53 and Hypoxia Signaling Pathways in Cancer.Front Cell Dev Biol. 2021 Feb 18;9:648808. doi: 10.3389/fcell.2021.648808. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681231 Free PMC article. Review.
-
Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency.Cell Stem Cell. 2014 May 1;14(5):592-605. doi: 10.1016/j.stem.2014.02.012. Epub 2014 Mar 20. Cell Stem Cell. 2014. PMID: 24656769 Free PMC article.
-
N-(2-mercaptopropionyl)-glycine enhances in vitro pig embryo production and reduces oxidative stress.Sci Rep. 2020 Oct 29;10(1):18632. doi: 10.1038/s41598-020-75442-6. Sci Rep. 2020. PMID: 33122658 Free PMC article.
-
Prolyl Hydroxylase Domain Protein Inhibitor Not Harboring a 2-Oxoglutarate Scaffold Protects against Hypoxic Stress.ACS Pharmacol Transl Sci. 2022 Apr 13;5(5):362-372. doi: 10.1021/acsptsci.2c00002. eCollection 2022 May 13. ACS Pharmacol Transl Sci. 2022. PMID: 35592438 Free PMC article.
-
The Next-Generation of Combination Cancer Immunotherapy: Epigenetic Immunomodulators Transmogrify Immune Training to Enhance Immunotherapy.Cancers (Basel). 2021 Jul 18;13(14):3596. doi: 10.3390/cancers13143596. Cancers (Basel). 2021. PMID: 34298809 Free PMC article. Review.
References
-
- Kim WS, Park BS, Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–887. - PubMed
-
- Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. - PubMed
-
- An WG, Kanekal M, Simon MC, et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

