Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access
- PMID: 22689651
- PMCID: PMC3373398
- DOI: 10.1083/jcb.201111146
Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access
Abstract
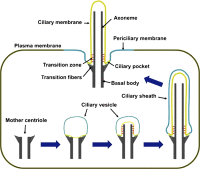
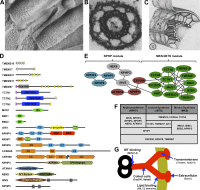
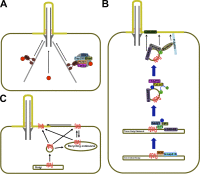
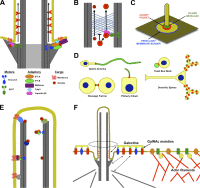
Cilia are conserved, microtubule-based cell surface projections that emanate from basal bodies, membrane-docked centrioles. The beating of motile cilia and flagella enables cells to swim and epithelia to displace fluids. In contrast, most primary cilia do not beat but instead detect environmental or intercellular stimuli. Inborn defects in both kinds of cilia cause human ciliopathies, diseases with diverse manifestations such as heterotaxia and kidney cysts. These diseases are caused by defects in ciliogenesis or ciliary function. The signaling functions of cilia require regulation of ciliary composition, which depends on the control of protein traffic into and out of cilia.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

