Integrity of the P-site is probed during maturation of the 60S ribosomal subunit
- PMID: 22689654
- PMCID: PMC3373404
- DOI: 10.1083/jcb.201112131
Integrity of the P-site is probed during maturation of the 60S ribosomal subunit
Abstract
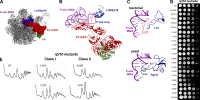
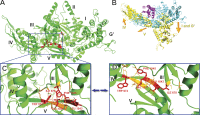
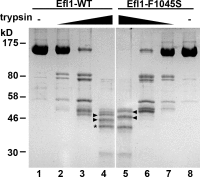
Eukaryotic ribosomes are preassembled in the nucleus and mature in the cytoplasm. Release of the antiassociation factor Tif6 by the translocase-like guanosine triphosphatase Efl1 is a critical late maturation step. In this paper, we show that a loop of Rpl10 that embraces the P-site transfer ribonucleic acid was required for release of Tif6, 90 Å away. Mutations in this P-site loop blocked 60S maturation but were suppressed by mutations in Tif6 or Efl1. Molecular dynamics simulations of the mutant Efl1 proteins suggest that they promote a conformation change in Efl1 equivalent to changes that elongation factor G and eEF2 undergo during translocation. These results identify molecular signaling from the P-site to Tif6 via Efl1, suggesting that the integrity of the P-site is interrogated during maturation. We propose that Efl1 promotes a functional check of the integrity of the 60S subunit before its first round of translation.
Figures





Similar articles
-
Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit.Mol Cell. 2010 Jul 30;39(2):196-208. doi: 10.1016/j.molcel.2010.06.018. Mol Cell. 2010. PMID: 20670889 Free PMC article.
-
Mutations in EFL1, an SBDS partner, are associated with infantile pancytopenia, exocrine pancreatic insufficiency and skeletal anomalies in aShwachman-Diamond like syndrome.J Med Genet. 2017 Aug;54(8):558-566. doi: 10.1136/jmedgenet-2016-104366. Epub 2017 Mar 22. J Med Genet. 2017. PMID: 28331068
-
The T-cell leukemia related rpl10-R98S mutant traps the 60S export adapter Nmd3 in the ribosomal P site in yeast.PLoS Genet. 2017 Jul 17;13(7):e1006894. doi: 10.1371/journal.pgen.1006894. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28715419 Free PMC article.
-
Insights into remodeling events during eukaryotic large ribosomal subunit assembly provided by high resolution cryo-EM structures.RNA Biol. 2017 Oct 3;14(10):1306-1313. doi: 10.1080/15476286.2017.1297914. Epub 2017 Mar 7. RNA Biol. 2017. PMID: 28267408 Free PMC article. Review.
-
Joining the interface: a site for Nmd3 association on 60S ribosome subunits.J Cell Biol. 2010 Jun 28;189(7):1071-3. doi: 10.1083/jcb.201006033. J Cell Biol. 2010. PMID: 20584913 Free PMC article. Review.
Cited by
-
The Roles of Puf6 and Loc1 in 60S Biogenesis Are Interdependent, and Both Are Required for Efficient Accommodation of Rpl43.J Biol Chem. 2016 Sep 9;291(37):19312-23. doi: 10.1074/jbc.M116.732800. Epub 2016 Jul 25. J Biol Chem. 2016. PMID: 27458021 Free PMC article.
-
Nmd3 is a structural mimic of eIF5A, and activates the cpGTPase Lsg1 during 60S ribosome biogenesis.EMBO J. 2017 Apr 3;36(7):854-868. doi: 10.15252/embj.201696012. Epub 2017 Feb 8. EMBO J. 2017. PMID: 28179369 Free PMC article.
-
The evolutionary and functional diversity of classical and lesser-known cytoplasmic and organellar translational GTPases across the tree of life.BMC Genomics. 2015 Feb 14;16(1):78. doi: 10.1186/s12864-015-1289-7. BMC Genomics. 2015. PMID: 25756599 Free PMC article.
-
Coupled GTPase and remodelling ATPase activities form a checkpoint for ribosome export.Nature. 2014 Jan 2;505(7481):112-116. doi: 10.1038/nature12731. Epub 2013 Nov 17. Nature. 2014. PMID: 24240281 Free PMC article.
-
Eukaryotic rpL10 drives ribosomal rotation.Nucleic Acids Res. 2014 Feb;42(3):2049-63. doi: 10.1093/nar/gkt1107. Epub 2013 Nov 8. Nucleic Acids Res. 2014. PMID: 24214990 Free PMC article.
References
-
- Armache J.P., Jarasch A., Anger A.M., Villa E., Becker T., Bhushan S., Jossinet F., Habeck M., Dindar G., Franckenberg S., et al. 2010. Localization of eukaryote-specific ribosomal proteins in a 5.5-Å cryo-EM map of the 80S eukaryotic ribosome. Proc. Natl. Acad. Sci. USA. 107:19754–19759 10.1073/pnas.1010005107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

