The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking
- PMID: 22689656
- PMCID: PMC3373400
- DOI: 10.1083/jcb.201110049
The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking
Abstract
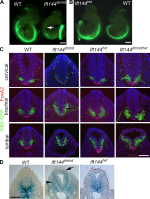
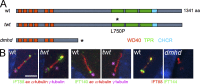
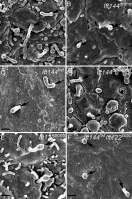
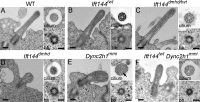
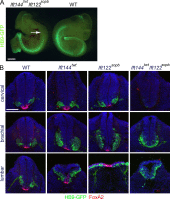
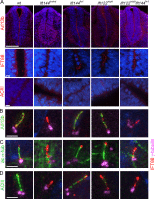
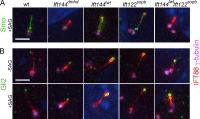
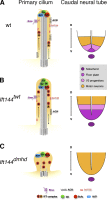
Two intraflagellar transport (IFT) complexes, IFT-A and IFT-B, build and maintain primary cilia and are required for activity of the Sonic hedgehog (Shh) pathway. A weak allele of the IFT-A gene, Ift144, caused subtle defects in cilia structure and ectopic activation of the Shh pathway. In contrast, strong loss of IFT-A, caused by either absence of Ift144 or mutations in two IFT-A genes, blocked normal ciliogenesis and decreased Shh signaling. In strong IFT-A mutants, the Shh pathway proteins Gli2, Sufu, and Kif7 localized correctly to cilia tips, suggesting that these pathway components were trafficked by IFT-B. In contrast, the membrane proteins Arl13b, ACIII, and Smo failed to localize to primary cilia in the absence of IFT-A. We propose that the increased Shh activity seen in partial loss-of-function IFT-A mutants may be a result of decreased ciliary ACIII and that the loss of Shh activity in the absence of IFT-A is a result of severe disruptions of cilia structure and membrane protein trafficking.
Figures
References
-
- Ashe A., Butterfield N.C., Town L., Courtney A.D., Cooper A.N., Ferguson C., Barry R., Olsson F., Liem K.F., Jr., Parton R.G., et al. 2012. Mutations in mouse Ift144 model the craniofacial, limb and rib defects in skeletal ciliopathies. Hum. Mol. Genet. 21:1808–1823 10.1093/hmg/ddr613 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

