The role of WDR5 in silencing human fetal globin gene expression
- PMID: 22689669
- PMCID: PMC3487434
- DOI: 10.3324/haematol.2012.061937
The role of WDR5 in silencing human fetal globin gene expression
Abstract
Background: Histone H3 lysine 4 (K4) methylation has been linked with transcriptional activity in mammalian cells. The WD40-repeat protein, WDR5, is an essential component of the MLL complex that induces histone H3 K4 methylation, but the role of WDR5 in human globin gene regulation has not yet been established.
Design and methods: To study the role of WDR5 in human globin gene regulation, we performed knockdown experiments in both K562 cells and primary human bone marrow erythroid progenitor cells (BMC). The effects of WDR5 knockdown on γ-globin gene expression were determined. Biochemical approaches were also employed to investigate WDR5 interaction molecules. Chromosomal marks in the globin locus were analyzed by ChIP.
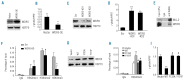
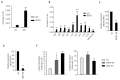
Results: We found that WDR5 interacted with protein arginine methyltransferase 5 (PRMT5), a known repressor of γ-globin gene expression, and was essential for generating tri-methylated H3K4 (H3K4me3) at the γ-globin promoter in K562 cells. Enforced expression of WDR5 in K562 cells reduced γ-globin gene expression, whereas knockdown of WDR5 increased γ-globin gene expression in both K562 cells and primary human bone marrow erythroid progenitor cells. Consistent with this, both histone H3 and H4 acetylation at the γ-globin promoter were increased, while histone H4R3 and H3K9 methylation were decreased, in WDR5 knockdown cells compared to controls. We found that WDR5 interacted with HDAC1 and a PHD domaincontaining protein, ING2 (inhibitor of growth), an H3K4me3 mark reader, to enhance γ-globin gene transcriptional repression. In human BMC, levels of WDR5 were highly enriched on the γ-promoter relative to levels on other globin promoters and compared to the γ-promoter in cord blood erythroid progenitors, suggesting that WDR5 is important in the developmental globin gene expression program.
Conclusions: Our data are consistent with a model in which WDR5 binds the γ-globin promoter in a PRMT5-dependent manner; H3K4me3 induced at the γ-globin promoter by WDR5 may result in the recruitment of the ING2-associated HDAC1 component and consequent silencing of γ-globin gene expression.
Figures



Similar articles
-
Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression.Blood. 2010 Sep 2;116(9):1585-92. doi: 10.1182/blood-2009-10-251116. Epub 2010 May 21. Blood. 2010. PMID: 20495075 Free PMC article.
-
Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive.Nature. 2007 Oct 18;449(7164):933-7. doi: 10.1038/nature06166. Epub 2007 Sep 26. Nature. 2007. PMID: 17898714
-
Histone arginine methylation keeps RUNX1 target genes in an intermediate state.Oncogene. 2013 May 16;32(20):2565-75. doi: 10.1038/onc.2012.274. Epub 2012 Jul 9. Oncogene. 2013. PMID: 22777353
-
On WD40 proteins: propelling our knowledge of transcriptional control?Epigenetics. 2012 Aug;7(8):815-22. doi: 10.4161/epi.21140. Epub 2012 Jul 19. Epigenetics. 2012. PMID: 22810296 Free PMC article. Review.
-
Plant homeodomain fingers form a helping hand for transcription.Epigenetics. 2011 Jan;6(1):4-8. doi: 10.4161/epi.6.1.13297. Epub 2011 Jan 1. Epigenetics. 2011. PMID: 20818169 Free PMC article. Review.
Cited by
-
Inhibitor of Growth Proteins: Epigenetic Regulators Shaping Neurobiology.Biomolecules. 2025 Feb 14;15(2):281. doi: 10.3390/biom15020281. Biomolecules. 2025. PMID: 40001584 Free PMC article. Review.
-
Transcriptional silencing of fetal hemoglobin expression by NonO.Nucleic Acids Res. 2021 Sep 27;49(17):9711-9723. doi: 10.1093/nar/gkab671. Nucleic Acids Res. 2021. PMID: 34379783 Free PMC article.
-
PRMT5: a putative oncogene and therapeutic target in prostate cancer.Cancer Gene Ther. 2022 Mar;29(3-4):264-276. doi: 10.1038/s41417-021-00327-3. Epub 2021 Apr 14. Cancer Gene Ther. 2022. PMID: 33854218 Free PMC article. Review.
-
Heterochromatin Protein 1γ Is a Novel Epigenetic Repressor of Human Embryonic ϵ-Globin Gene Expression.J Biol Chem. 2017 Mar 24;292(12):4811-4817. doi: 10.1074/jbc.M116.768515. Epub 2017 Feb 1. J Biol Chem. 2017. PMID: 28154185 Free PMC article.
-
WDR5 facilitates EMT and metastasis of CCA by increasing HIF-1α accumulation in Myc-dependent and independent pathways.Mol Ther. 2021 Jun 2;29(6):2134-2150. doi: 10.1016/j.ymthe.2021.02.017. Epub 2021 Feb 15. Mol Ther. 2021. PMID: 33601056 Free PMC article.
References
-
- Ginder GD, Gnanapragasam MN, Mian OY. The role of the epigenetic signal, DNA methylation, in gene regulation during erythroid development. Curr Top Dev Biol. 2008;82:85-116 - PubMed
-
- Atweh GF, DeSimone J, Saunthararajah Y, Fathallah H, Weinberg RS, Nagel RL, et al. Hemoglobinopathies. Hematology Am Soc Hematol Educ Program. 2003:14-39 - PubMed
-
- Perrine SP. Fetal globin stimulant therapies in the beta-hemoglobinopathies: principles and current potential. Pediatr Ann. 2008;37(5):339-46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

