Anti-CD20 IgA can protect mice against lymphoma development: evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells
- PMID: 22689689
- PMCID: PMC3487441
- DOI: 10.3324/haematol.2011.061408
Anti-CD20 IgA can protect mice against lymphoma development: evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells
Abstract
Background: While most antibody-based therapies use IgG because of their well-known biological properties, some functional limitations of these antibodies call for the development of derivatives with other therapeutic functions. Although less abundant than IgG in serum, IgA is the most abundantly produced Ig class in humans. Besides the specific targeting of its dimeric form to mucosal areas, IgA was shown to recruit polymorphonuclear neutrophils against certain targets more efficiently than does IgG1.
Design and methods: In this study, we investigated the various pathways by which anti-tumor effects can be mediated by anti-CD20 IgA against lymphoma cells.
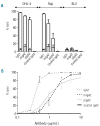
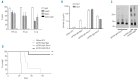
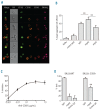
Results: We found that polymeric human IgA was significantly more effective than human IgG1 in mediating direct killing or growth inhibition of target cells in the absence of complement. We also demonstrated that this direct killing was able to indirectly induce the classical pathway of the complement cascade although to a lesser extent than direct recruitment of complement by IgG. Recruitment of the alternative complement pathway by specific IgA was also observed. In addition to activating complement for lysis of lymphoma cell lines or primary cells from patients with lymphoma, we showed that monomeric anti-CD20 IgA can effectively protect mice against tumor development in a passive immunization strategy and we demonstrated that this protective effect may be enhanced in mice expressing the human FcαRI receptor on their neutrophils.
Conclusions: We show that anti-CD20 IgA antibodies have original therapeutic properties against lymphoma cells, with strong direct effects, ability to recruit neutrophils for cell cytotoxicity and even recruitment of complement, although largely through an indirect way.
Figures


References
-
- Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class Irelated receptor FcRn. Annu Rev Immunol. 2000;18739-66 - PubMed
-
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;1263-84 - PubMed
-
- Daha MR, Gorter A, Rits M, van Es LA, Hiemstra PS. Interaction of immunoglobulin A with complement and phagocytic cells. Prog Clin Biol Res. 1989;297:247-60; discussion 60-1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

