Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia
- PMID: 22689805
- PMCID: PMC4874148
- DOI: 10.1200/JCO.2011.38.9429
Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia
Abstract
Purpose: This multicenter, randomized, open-label, phase III trial compared the efficacy and safety of decitabine with treatment choice (TC) in older patients with newly diagnosed acute myeloid leukemia (AML) and poor- or intermediate-risk cytogenetics.
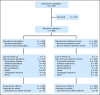
Patients and methods: Patients (N = 485) age ≥ 65 years were randomly assigned 1:1 to receive decitabine 20 mg/m(2) per day as a 1-hour intravenous infusion for five consecutive days every 4 weeks or TC (supportive care or cytarabine 20 mg/m(2) per day as a subcutaneous injection for 10 consecutive days every 4 weeks). The primary end point was overall survival (OS); the secondary end point was the complete remission (CR) rate plus the CR rate without platelet recovery (CRp). Adverse events (AEs) were recorded.
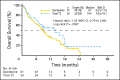
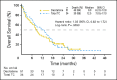
Results: The primary analysis with 396 deaths (81.6%) showed a nonsignificant increase in median OS with decitabine (7.7 months; 95% CI, 6.2 to 9.2) versus TC (5.0 months; 95% CI, 4.3 to 6.3; P = .108; hazard ratio [HR], 0.85; 95% CI, 0.69 to 1.04). An unplanned analysis with 446 deaths (92%) indicated the same median OS (HR, 0.82; 95% CI, 0.68 to 0.99; nominal P = .037). The CR rate plus CRp was 17.8% with decitabine versus 7.8% with TC (odds ratio, 2.5; 95% CI, 1.4 to 4.8; P = .001). AEs were similar for decitabine and cytarabine, although patients received a median of four cycles of decitabine versus two cycles of TC. The most common drug-related AEs with decitabine were thrombocytopenia (27%) and neutropenia (24%).
Conclusion: In older patients with AML, decitabine improved response rates compared with standard therapies without major differences in safety. An unplanned survival analysis showed a benefit for decitabine, which was not observed at the time of the primary analysis.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
What is better for older patients with acute myeloid leukemia?J Clin Oncol. 2013 Feb 20;31(6):820-1. doi: 10.1200/JCO.2012.45.2219. Epub 2012 Nov 5. J Clin Oncol. 2013. PMID: 23129743 No abstract available.
-
When azanucleoside treatment can be curative: nonintensive bridging strategy before allografting in older patients with myelodysplastic syndrome/acute myeloid leukemia.J Clin Oncol. 2013 Feb 20;31(6):822-3. doi: 10.1200/JCO.2012.46.4222. Epub 2012 Nov 5. J Clin Oncol. 2013. PMID: 23129745 No abstract available.
References
-
- American Cancer Society. Cancer facts and figures. 2010. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume....
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(suppl 1):i3–i8. - PubMed
-
- National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology. Acute myeloid leukemia v 2. 2011. http://guidelines.nccn.org/epc-guideline/guideline/id/F4F85BCF-EB5A-32AC....
-
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

