Sequential Plasmodium chabaudi and Plasmodium berghei infections provide a novel model of severe malarial anemia
- PMID: 22689817
- PMCID: PMC3418732
- DOI: 10.1128/IAI.06185-11
Sequential Plasmodium chabaudi and Plasmodium berghei infections provide a novel model of severe malarial anemia
Abstract
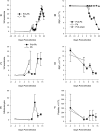
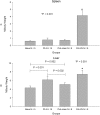
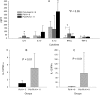
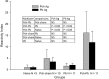
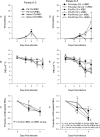
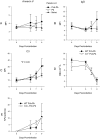
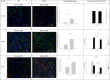
Lack of an adequate animal model of Plasmodium falciparum severe malarial anemia (SMA) has hampered the understanding of this highly lethal condition. We developed a model of SMA by infecting C57BL/6 mice with P. chabaudi followed after recovery by P. berghei infection. P. chabaudi/P. berghei-infected mice had an initial 9- to 10-day phase of relatively low parasitemia and severe anemia, followed by a second phase of hyperparasitemia, more profound anemia, reticulocytosis, and death 14 to 21 days after infection. P. chabaudi/P. berghei-infected animals had more intense splenic hematopoiesis, higher interleukin-10 (IL-10)/tumor necrosis factor alpha and IL-12/gamma interferon (IFN-γ) ratios, and higher antibody levels against P. berghei and P. chabaudi antigens than P. berghei-infected or P. chabaudi-recovered animals. Early treatment with chloroquine or artesunate did not prevent the anemia, suggesting that the bulk of red cell destruction was not due to the parasite. Red cells from P. chabaudi/P. berghei-infected animals had increased surface IgG and C3 by flow cytometry. However, C3(-/-) mice still developed anemia. Tracking of red cells labeled ex vivo and in vivo and analysis of frozen tissue sections by immunofluorescence microscopy showed that red cells from P. chabaudi/P. berghei-infected animals were removed at an accelerated rate in the liver by erythrophagocytosis. This model is practical and reproducible, and its similarities with P. falciparum SMA in humans makes it an appealing system with which to study the pathogenesis of this condition and explore potential immunomodulatory interventions.
Figures
References
-
- Abdalla SH, Weatherall DJ, Wickramasinghe SN, Hughes M. 1980. The anaemia of P. falciparum malaria. Br. J. Haematol. 46:171–183 - PubMed
-
- Akanmori BD, et al. 2000. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur. Cytokine Netw. 11:113–118 - PubMed
-
- Camacho LH, et al. 1998. The course of anaemia after the treatment of acute, falciparum malaria. Ann. Trop. Med. Parasitol. 92:525–537 - PubMed
-
- Chang KH, Tam M, Stevenson MM. 2004. Modulation of the course and outcome of blood-stage malaria by erythropoietin-induced reticulocytosis. J. Infect. Dis. 189:735–743 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

