E6AP ubiquitin ligase regulates PML-induced senescence in Myc-driven lymphomagenesis
- PMID: 22689861
- PMCID: PMC3709628
- DOI: 10.1182/blood-2011-10-387647
E6AP ubiquitin ligase regulates PML-induced senescence in Myc-driven lymphomagenesis
Erratum in
- Blood. 2014 Aug 14;124(7):1200
Abstract
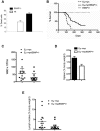
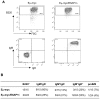
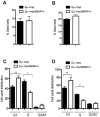
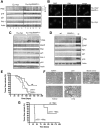
Neoplastic transformation requires the elimination of key tumor suppressors, which may result from E3 ligase-mediated proteasomal degradation. We previously demonstrated a key role for the E3 ubiquitin ligase E6AP in the regulation of promyelocytic leukemia protein (PML) stability and formation of PML nuclear bodies. Here, we report the involvement of the E6AP-PML axis in B-cell lymphoma development. A partial loss of E6AP attenuated Myc-induced B-cell lymphomagenesis. This tumor suppressive action was achieved by the induction of cellular senescence. B-cell lymphomas deficient for E6AP expressed elevated levels of PML and PML-nuclear bodies with a concomitant increase in markers of cellular senescence, including p21, H3K9me3, and p16. Consistently, PML deficiency accelerated the rate of Myc-induced B-cell lymphomagenesis. Importantly, E6AP expression was elevated in ∼ 60% of human Burkitt lymphomas, and down-regulation of E6AP in B-lymphoma cells restored PML expression with a concurrent induction of cellular senescence in these cells. Our findings demonstrate that E6AP-mediated down-regulation of PML-induced senescence is essential for B-cell lymphoma progression. This provides a molecular explanation for the down-regulation of PML observed in non-Hodgkin lymphomas, thereby suggesting a novel therapeutic approach for restoration of tumor suppression in B-cell lymphoma.
Figures



References
-
- Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest. 2004;22(2):304–311. - PubMed
-
- Castéra L, Sabbagh A, Dehainault C, et al. MDM2 as a modifier gene in retinoblastoma. J Natl Cancer Inst. 2010;102(23):1805–1808. - PubMed
-
- Leach FS, Tokino T, Meltzer P, et al. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53(10 Suppl):2231–2234. - PubMed
-
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358(6381):80–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

