Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma
- PMID: 22689881
- PMCID: PMC3393638
- DOI: 10.4049/jimmunol.1103641
Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma
Abstract
Vascular endothelial growth factor (VEGF), a key angiogenic molecule, is aberrantly expressed in several diseases including asthma where it contributes to bronchial vascular remodeling and chronic inflammation. Asthmatic human airway smooth muscle cells hypersecrete VEGF, but the mechanism is unclear. In this study, we defined the mechanism in human airway smooth muscle cells from nonasthmatic and asthmatic patients. We found that asthmatic cells lacked a repression complex at the VEGF promoter, which was present in nonasthmatic cells. Recruitment of G9A, trimethylation of histone H3 at lysine 9 (H3K9me3), and a resultant decrease in RNA polymerase II at the VEGF promoter was critical to repression of VEGF secretion in nonasthmatic cells. At the asthmatic promoter, H3K9me3 was absent because of failed recruitment of G9a; RNA polymerase II binding, in association with TATA-binding protein-associated factor 1, was increased; H3K4me3 was present; and Sp1 binding was exaggerated and sustained. In contrast, DNA methylation and histone acetylation were similar in asthmatic and nonasthmatic cells. This is the first study, to our knowledge, to show that airway cells in asthma have altered epigenetic regulation of remodeling gene(s). Histone methylation at genes such as VEGF may be an important new therapeutic target.
Figures
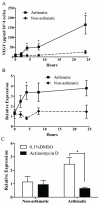
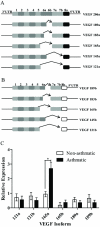
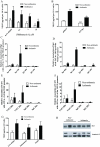

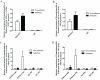
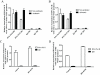
 = site methylated in at least one clone. (E) VEGF protein levels after incubation with 5-aza. N = 4 NA and 3 A HASM cell lines.
= site methylated in at least one clone. (E) VEGF protein levels after incubation with 5-aza. N = 4 NA and 3 A HASM cell lines.



References
-
- Kazi AS, Lotfi S, Goncharova EA, Tliba O, Amrani Y, Krymskaya VP, Lazaar AL. Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am J Physiol Lung Cell Mol Physiol. 2004;286:L539–545. - PubMed
-
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. American journal of respiratory and critical care medicine. 2001;164:S39–45. - PubMed
-
- Lee SY, Kwon S, Kim KH, Moon HS, Song JS, Park SH, Kim YK. Expression of vascular endothelial growth factor and hypoxia-inducible factor in the airway of asthmatic patients. Ann Allergy Asthma Immunol. 2006;97:794–799. - PubMed
-
- Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy. 2003;33:595–599. - PubMed
-
- Siddiqui S, Sutcliffe A, Shikotra A, Woodman L, Doe C, McKenna S, Wardlaw A, Bradding P, Pavord I, Brightling C. Vascular remodeling is a feature of asthma and nonasthmatic eosinophilic bronchitis. J Allergy Clin Immunol. 2007;120:813–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

