Memantine and cholinesterase inhibitor combination therapy for Alzheimer's disease: a systematic review
- PMID: 22689908
- PMCID: PMC3378937
- DOI: 10.1136/bmjopen-2012-000917
Memantine and cholinesterase inhibitor combination therapy for Alzheimer's disease: a systematic review
Abstract
Background: Memantine is licensed for moderate-to-severe Alzheimer's disease (AD). National Institute for Clinical Excellence (NICE) guidance does not recommend the use of memantine in combination with cholinesterase inhibitors (acetylcholinesterase inhibitor (AChEI)). The underpinning meta-analysis was disputed by the manufacturer.
Objectives: To compare the efficacy of AChEI monotherapy with combination memantine and AChEI therapy in patients with moderate-to-severe AD and to examine the impact of including unpublished data on the results.
Design: Systematic review and meta-analysis of randomised controlled trials.
Data sources: The Cochrane Dementia Group trial register, ALOIS, searched for the last time on 3 May 2011.
Data synthesis: Data from four domains (clinical global, cognition, function, behaviour and mood) were pooled. Sensitivity analyses examined the impact on the NICE-commissioned meta-analysis of restricting data to patients with moderate-to-severe AD and of including an unpublished trial of an extended release preparation of memantine.
Results: Pooled data from the trials, which were included in the NICE-commissioned meta-analysis but which were restricted to moderate-to-severe AD only, showed a small effect of combination therapy on cognition (standardised mean difference (SMD)=-0.29, 95% CI -0.45 to -0.14). Adding data from an unpublished trial of an extended release memantine (total three trials, 1317 participants) showed a small benefit of combination therapy on global scores (SMD=-0.20, 95% CI -0.31 to -0.09), cognition (SMD=-0.25, 95% CI -0.36 to -0.14) and behaviour and mood (SMD=-0.17, 95% CI -0.32 to -0.03) but not on function (SMD=-0.04, 95% CI -0.21 to 0.13) at 6 months. No clinical data have been reported from a 1-year trial, although this found 'no significant benefit' on any clinical measures at 1 year.
Conclusions: These results suggest that there may be a small benefit at 6 months of adding memantine to AChEIs. However, the impact on clinical global impression depends on exactly which studies are included, and there is no benefit on function, so its clinical relevance is not robustly demonstrated. Currently available information from randomised controlled trails indicates no benefit of combination therapy over monotherapy at 1 year. Legislation on the form and content of registry posted results is needed in Europe.
Conflict of interest statement
Figures
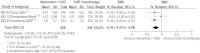



References
-
- EMEA Committee for Medicinal Products for Human Use October 2005 Plenary Meeting Monthly Report. European Medicines Agency Website. http://www.emea.europa.eu/pdfs/human/press/pr/36234805en.pdf (accessed 17 Nov 2005).
-
- National Institute for Clinical Excellence (NICE) Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer's Disease. NICE Technology Appraisal Guidance 217 (Review of NICE Technology Appraisal Guidance 111). National Institute for Clinical Excellence, 2011. http://publications.nice.org.uk/donepezil-galantamine-rivastigmine-and-m... (accessed 23 Mar 2012).
-
- Institute for Quality and Efficiency in Healthcare (IQWiG) Memantine in Alzheimer's Disease. Executive Summary of Final Report A05–19C. Institute for Quality and Efficiency in Healthcare (IQWiG), 2009. https://www.iqwig.de/download/A05-19C_Executive_Summary_Memantine_in_Alz... (Translation of the executive summary of the final report “Memantin bei Alzheimer Demenz”) (accessed 23 Mar 2012).
-
- Institute for Quality and Efficiency in Health Care (IQWiG) Federal Joint Committee. Responder Analyses on Memantine in Alzheimer's Disease: Executive Summary of Rapid Report A10–06. Institute for Quality and Efficiency in Healthcare (IQWiG), 2011. https://www.iqwig.de/download/A10-06_Executive-summary_Responder_analyse... (accessed 23 Mar 2012).
-
- Institute for Quality and Efficiency in Healthcare (IQWiG) Press Release: Memantine in Alzheimer's Disease: Reliable Analyses are Required. Institute for Quality and Efficiency in Healthcare (IQWiG), 2010. https://www.iqwig.de/memantine-in-alzheimer-s-disease-reliable.1079.en.html (accessed 23 Mar 2012).
LinkOut - more resources
Full Text Sources
Medical
