Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer
- PMID: 22689960
- PMCID: PMC3390855
- DOI: 10.1073/pnas.1207829109
Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer
Abstract
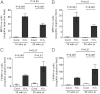
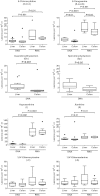
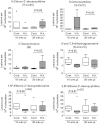
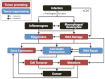
Helicobacter hepaticus-infected Rag2(-/-) mice emulate many aspects of human inflammatory bowel disease, including the development of colitis and colon cancer. To elucidate mechanisms of inflammation-induced carcinogenesis, we undertook a comprehensive analysis of histopathology, molecular damage, and gene expression changes during disease progression in these mice. Infected mice developed severe colitis and hepatitis by 10 wk post-infection, progressing into colon carcinoma by 20 wk post-infection, with pronounced pathology in the cecum and proximal colon marked by infiltration of neutrophils and macrophages. Transcriptional profiling revealed decreased expression of DNA repair and oxidative stress response genes in colon, but not in liver. Mass spectrometric analysis revealed higher levels of DNA and RNA damage products in liver compared to colon and infection-induced increases in 5-chlorocytosine in DNA and RNA and hypoxanthine in DNA. Paradoxically, infection was associated with decreased levels of DNA etheno adducts. Levels of nucleic acid damage from the same chemical class were strongly correlated in both liver and colon. The results support a model of inflammation-mediated carcinogenesis involving infiltration of phagocytes and generation of reactive species that cause local molecular damage leading to cell dysfunction, mutation, and cell death. There are strong correlations among histopathology, phagocyte infiltration, and damage chemistry that suggest a major role for neutrophils in inflammation-associated cancer progression. Further, paradoxical changes in nucleic acid damage were observed in tissue- and chemistry-specific patterns. The results also reveal features of cell stress response that point to microbial pathophysiology and mechanisms of cell senescence as important mechanistic links to cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dale DC, Boxer L, Liles WC. The phagocytes: Neutrophils and monocytes. Blood. 2008;112:935–945. - PubMed
-
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. - PubMed
-
- Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

