Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action
- PMID: 22689970
- PMCID: PMC3387046
- DOI: 10.1073/pnas.1116590109
Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action
Abstract
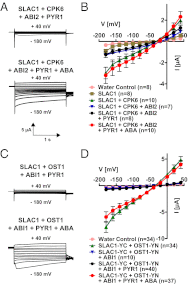
The plant hormone abscisic acid (ABA) is produced in response to abiotic stresses and mediates stomatal closure in response to drought via recently identified ABA receptors (pyrabactin resistance/regulatory component of ABA receptor; PYR/RCAR). SLAC1 encodes a central guard cell S-type anion channel that mediates ABA-induced stomatal closure. Coexpression of the calcium-dependent protein kinase 21 (CPK21), CPK23, or the Open Stomata 1 kinase (OST1) activates SLAC1 anion currents. However, reconstitution of ABA activation of any plant ion channel has not yet been attained. Whether the known core ABA signaling components are sufficient for ABA activation of SLAC1 anion channels or whether additional components are required remains unknown. The Ca(2+)-dependent protein kinase CPK6 is known to function in vivo in ABA-induced stomatal closure. Here we show that CPK6 robustly activates SLAC1-mediated currents and phosphorylates the SLAC1 N terminus. A phosphorylation site (S59) in SLAC1, crucial for CPK6 activation, was identified. The group A PP2Cs ABI1, ABI2, and PP2CA down-regulated CPK6-mediated SLAC1 activity in oocytes. Unexpectedly, ABI1 directly dephosphorylated the N terminus of SLAC1, indicating an alternate branched early ABA signaling core in which ABI1 targets SLAC1 directly (down-regulation). Furthermore, here we have successfully reconstituted ABA-induced activation of SLAC1 channels in oocytes using the ABA receptor pyrabactin resistant 1 (PYR1) and PP2C phosphatases with two alternate signaling cores including either CPK6 or OST1. Point mutations in ABI1 disrupting PYR1-ABI1 interaction abolished ABA signal transduction. Moreover, by addition of CPK6, a functional ABA signal transduction core from ABA receptors to ion channel activation was reconstituted without a SnRK2 kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ma Y, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. - PubMed
-
- Santiago J, et al. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009;60:575–588. - PubMed
-
- Szostkiewicz I, et al. Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J. 2010;61(1):25–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

