Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo
- PMID: 22689978
- PMCID: PMC3387068
- DOI: 10.1073/pnas.1201131109
Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo
Abstract
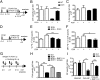
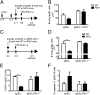
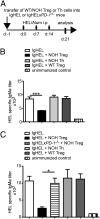
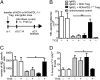
The mechanisms by which regulatory T cells (T(regs)) suppress autoantibody production are unclear. Here we have addressed this question using transgenic mice expressing model antigens in the kidney. We report that T(regs) were essential and sufficient to suppress autoreactive B cells in an antigen-specific manner and to prevent them from producing autoantibodies. Most of this suppression was mediated through the inhibitory cell-surface-molecule programmed death-1 (PD-1). Suppression required PD-1 expression on autoreactive B cells and expression of the two PD-1 ligands on T(regs). PD-1 ligation inhibited activation of autoreactive B cells, suppressed their proliferation, and induced their apoptosis. Intermediate PD-1(+) cells, such as T helper cells, were dispensable for suppression. These findings demonstrate in vivo that T(regs) use PD-1 ligands to directly suppress autoreactive B cells, and they identify a previously undescribed peripheral B-cell tolerance mechanism against tissue autoantigens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. - PubMed
-
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. - PubMed
-
- Seo SJ, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

