Opposing roles for RhoH GTPase during T-cell migration and activation
- PMID: 22689994
- PMCID: PMC3387109
- DOI: 10.1073/pnas.1114214109
Opposing roles for RhoH GTPase during T-cell migration and activation
Abstract
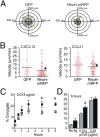
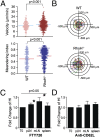
T cells spend the majority of their time perusing lymphoid organs in search of cognate antigen presented by antigen presenting cells (APCs) and then quickly recirculate through the bloodstream to another lymph node. Therefore, regulation of a T-cell response is dependent upon the ability of cells to arrive in the correct location following chemokine gradients ("go" signal) as well as to receive appropriate T-cell receptor (TCR) activation signals upon cognate antigen recognition ("stop" signal). However, the mechanisms by which T cells regulate these go and stop signals remain unclear. We found that overexpression of the hematopoietic-specific RhoH protein in the presence of chemokine signals resulted in decreased Rap1-GTP and LFA-1 adhesiveness to ICAM-1, thus impairing T-cell chemotaxis; while in the presence of TCR signals, there were enhanced and sustained Rap1-GTP and LFA-1 activation as well as prolonged T:APC conjugates. RT-PCR analyses of activated CD4(+) T cells and live images of T-cell migration and immunological synapse (IS) formation revealed that functions of RhoH took place primarily at the levels of transcription and intracellular distribution. Thus, we conclude that RhoH expression provides a key molecular determinant that allows T cells to switch between sensing chemokine-mediated go signals and TCR-dependent stop signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. - PubMed
-
- Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: More than just sticking points. J Cell Sci. 2003;116:4695–4705. - PubMed
-
- Vigl B, et al. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. - PubMed
-
- Bromley SK, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. - PubMed
-
- Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5:363–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

