Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects?
- PMID: 22690666
- PMCID: PMC3970193
- DOI: 10.2217/epi.12.20
Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects?
Abstract
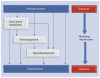
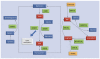
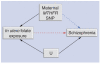
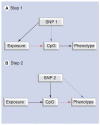
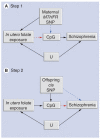
We posit that maternal prenatal nutrition can influence offspring schizophrenia risk via epigenetic effects. In this article, we consider evidence that prenatal nutrition is linked to epigenetic outcomes in offspring and schizophrenia in offspring, and that schizophrenia is associated with epigenetic changes. We focus upon one-carbon metabolism as a mediator of the pathway between perturbed prenatal nutrition and the subsequent risk of schizophrenia. Although post-mortem human studies demonstrate DNA methylation changes in brains of people with schizophrenia, such studies cannot establish causality. We suggest a testable hypothesis that utilizes a novel two-step Mendelian randomization approach, to test the component parts of the proposed causal pathway leading from prenatal nutritional exposure to schizophrenia. Applied here to a specific example, such an approach is applicable for wider use to strengthen causal inference of the mediating role of epigenetic factors linking exposures to health outcomes in population-based studies.
Figures
References
-
- Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch. Gen. Psychiatry. 1999;56:162–168. - PubMed
-
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am. J. Epidemiol. 2007;165:1–13. - PubMed
-
- Peerbooms OLJ, van Os J, Drukker M, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav. Immun. 2011;25:1530–1543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
