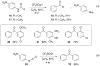
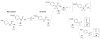Friedel-Crafts acylation with amides
- PMID: 22690740
- PMCID: PMC3402163
- DOI: 10.1021/jo300922p
Friedel-Crafts acylation with amides
Abstract
Friedel-Crafts acylation has been known since the 1870s, and it is an important organic synthetic reaction leading to aromatic ketone products. Friedel-Crafts acylation is usually performed with carboxylic acid chlorides or anhydrides, while amides are generally not useful substrates in these reactions. Despite being the least reactive carboxylic acid derivative, we have found a series of amides capable of providing aromatic ketones in good yields (55-96%, 17 examples). We propose a mechanism involving diminished C-N resonance through superelectrophilic activation and subsequent cleavage to acyl cations.
Figures
References
-
- Friedel C, Crafts JM. Compt Rend. 1877;84:1450.
-
- Gore PH. In: Friedel-Crafts and Related Reactions. Part I. Olah GA, editor. III. John Wiley & Sons Inc; London: 1964. p. 1.
-
- Sartori JM, Maggi R. Chem Rev. 2011;111:PR181–PR214. - PubMed
-
- Frank HG. Industrial Aromatic Chemistry. Springer; Berlin: 1988.
-
- Olah GA, White AM. J Am Chem Soc. 1967;89:7072.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources