Nonamorphism in flufenamic acid and a new record for a polymorphic compound with solved structures
- PMID: 22690822
- PMCID: PMC3634867
- DOI: 10.1021/ja302601f
Nonamorphism in flufenamic acid and a new record for a polymorphic compound with solved structures
Abstract
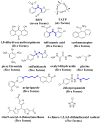
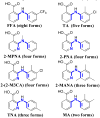
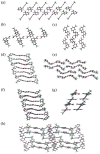
The unprecedented polymorphism of the non-steroidal anti-inflammatory drug (NSAID) flufenamic acid (FFA) is described here. Nine polymorphs were accessed through the use of polymer-induced heteronucleation (PIHn) and solid-solid transformation at low temperature. Structural elucidation of six of these forms, in addition to the two previously known forms, makes FFA indisputably octamorphic. Although the structure of at least one other form of FFA remains elusive, the occurrence of most of these polymorphs under one crystallization condition through PIHn illustrates that a fine interplay exists among the kinetic factors that lead to phase selection in this NSAID.
Figures
References
-
- McCrone WC. Physics and Chemistry of the Organic Solid State. Interscience Publishers; London: 1965.
-
- Platteau C, Lefebvre J, Hemon S, Baehtz C, Danede F, Prevost D. Acta Crystallogr. 2005;61:80. - PubMed
- Kato Y, Okamoto Y, Nagasawa S, Ueki T. Chem Pharm Bulletin. 1981;29:3410.
- Nikolics K, Bidlo G, Nikolics K. Acta Pharm. 1974;44:133. - PubMed
- Mesley RJ, Zlements RL. J Pharm Pharmac. 1968;20:341. - PubMed
-
-
Cited thereafter; Dixit G, Khile AS, Pradhan NS, Valgeirsson J. Patent 2009/007856. 2009
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

