Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer
- PMID: 22691503
- PMCID: PMC3375600
- DOI: 10.1097/RLU.0b013e318252d829
Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer
Abstract
Purpose: This study aimed to perform a prospective evaluation of 18F-NaF and 18F-FDG PET/CT in the detection of occult metastatic disease in men with prostate cancer and biochemical relapse.
Methods: Thirty-seven men with prostate-specific antigen (PSA) relapse (median, 3.2 ng/mL; range, 0.5-40.2 ng/mL) after definitive therapy for localized prostate cancer [26 radical prostatectomy (RP), 11 external beam radiation therapy] and negative conventional imaging underwent 18F-FDG and 18F-NaF PET/CT on 2 separate days within the same week. Studies were interpreted by 2 experienced radiologists in consensus for abnormal uptake suspicious for metastatic disease. The reference standard was a combination of imaging and clinical follow-up. Rank of PSA values for positive and negative PET/CT was compared using analysis of variance adjusting for primary therapy. Association between PSA and scan positivity in patients with RP was evaluated using Wilcoxon rank sum test.
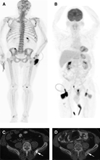
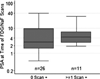
Results: Result of the 18F-FDG PET/CT scan was positive for nodal disease in 2 patients. True-positive detection rate for occult osseous metastases by 18F-NaF PET/CT was 16.2%. Median PSA levels for positive versus negative PET/CT scans were 4.4 and 2.9 ng/mL, respectively, with the difference marginally significant in prostatectomized men (P=0.072). Percentages of patients with either 18F-NaF- or 18F-FDG-positive PET/CT in RP and external beam radiation therapy were 10% (n=10) and undefined (n=0) for a PSA of 2 ng/mL or less, 29% (n=7) and 50% (n=2) for PSA greater than 2 ng/mL but 4 ng/mL or less, 60% (n=5) and 40% (n=5) for PSA greater than 4 ng/mL but 10 ng/mL or less, and 25% (n=4) and 25% (n=4) for PSA greater than 10 ng/mL, respectively.
Conclusions: In biochemical relapse of prostate cancer, 18 F-NaF PET/CT is useful in the detection of occult osseous metastases, whereas the yield of 18F-FDG PET/CT is relatively limited. 18F-NaF PET/CT positivity tends to associate with increasing PSA level in prostatectomized men and may occur in lower PSA ranges than conventionally recognized.
Conflict of interest statement
Conflicts of interest and sources of funding: none declared.
Figures



References
-
- National Cancer Institute. SEER: The Surveillance, Epidemiology, and End Results Program—based within the Surveillance Research Program at the National Cancer Institute. Baltimore, MD: National Cancer Institute; Available at: http://seer.cancer.gov.
-
- Dong JT, Rinker-Schaeffer CW, Ichikawa T, et al. Prostate cancer—biology of metastasis and its clinical implications. World J Urol. 1996;14:182–189. - PubMed
-
- Haseman MK, Rosenthal SA, Polascik TJ. Capromab pendetide imaging of prostate cancer. Cancer Biother Radiopharm. 2000;15:131–140. - PubMed
-
- Gillies RJ, Robey I, Catenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49:24S–42S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

