Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom
- PMID: 22691710
- PMCID: PMC9151880
- DOI: 10.1017/S0950268812001148
Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom
Abstract
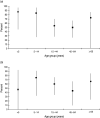
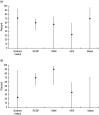
An analysis was undertaken to measure age-specific vaccine effectiveness (VE) of 2010/11 trivalent seasonal influenza vaccine (TIV) and monovalent 2009 pandemic influenza vaccine (PIV) administered in 2009/2010. The test-negative case-control study design was employed based on patients consulting primary care. Overall TIV effectiveness, adjusted for age and month, against confirmed influenza A(H1N1)pdm 2009 infection was 56% (95% CI 42-66); age-specific adjusted VE was 87% (95% CI 45-97) in <5-year-olds and 84% (95% CI 27-97) in 5- to 14-year-olds. Adjusted VE for PIV was only 28% (95% CI -6 to 51) overall and 72% (95% CI 15-91) in <5-year-olds. For confirmed influenza B infection, TIV effectiveness was 57% (95% CI 42-68) and in 5- to 14-year-olds 75% (95% CI 32-91). TIV provided moderate protection against the main circulating strains in 2010/2011, with higher protection in children. PIV administered during the previous season provided residual protection after 1 year, particularly in the <5 years age group.
Figures

Similar articles
-
End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18.Euro Surveill. 2019 Aug;24(31):1800488. doi: 10.2807/1560-7917.ES.2019.24.31.1800488. Euro Surveill. 2019. PMID: 31387673 Free PMC article.
-
Effectiveness of trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2012/13 end of season results.Euro Surveill. 2014 Jul 10;19(27):5-13. Euro Surveill. 2014. PMID: 25033051
-
Effectiveness of monovalent 2009 pandemic influenza A virus subtype H1N1 and 2010-2011 trivalent inactivated influenza vaccines in Wisconsin during the 2010-2011 influenza season.J Infect Dis. 2013 Apr 15;207(8):1262-9. doi: 10.1093/infdis/jit020. Epub 2013 Jan 22. J Infect Dis. 2013. PMID: 23341536
-
Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results.Euro Surveill. 2016 Sep 22;21(38):30348. doi: 10.2807/1560-7917.ES.2016.21.38.30348. Euro Surveill. 2016. PMID: 27684603 Free PMC article.
-
Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11.Euro Surveill. 2011 Feb 10;16(6):19791. Euro Surveill. 2011. PMID: 21329644
Cited by
-
Effectiveness of the 2010 and 2011 Southern Hemisphere trivalent inactivated influenza vaccines against hospitalization with influenza-associated acute respiratory infection among Thai adults aged ≥ 50 years.Influenza Other Respir Viruses. 2014 Jul;8(4):463-8. doi: 10.1111/irv.12233. Epub 2014 Feb 3. Influenza Other Respir Viruses. 2014. PMID: 24490684 Free PMC article.
-
Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study.PLoS Med. 2013 Nov;10(11):e1001558. doi: 10.1371/journal.pmed.1001558. Epub 2013 Nov 26. PLoS Med. 2013. PMID: 24302890 Free PMC article.
-
Epidemiological and health economic implications of symptom propagation in respiratory pathogens: A mathematical modelling investigation.PLoS Comput Biol. 2024 May 3;20(5):e1012096. doi: 10.1371/journal.pcbi.1012096. eCollection 2024 May. PLoS Comput Biol. 2024. PMID: 38701066 Free PMC article.
-
Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques.J Virol. 2013 May;87(10):5512-22. doi: 10.1128/JVI.03030-12. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468501 Free PMC article.
-
The Use of Test-negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of Methodology.Epidemiology. 2020 Jan;31(1):43-64. doi: 10.1097/EDE.0000000000001116. Epidemiology. 2020. PMID: 31609860 Free PMC article.
References
-
- Wichmann O, et al. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009–2010. Eurosurveillance 2010; 15. - PubMed
-
- Hardelid P, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009–2010. Eurosurveillance 2011; 16. - PubMed
-
- Pebody R, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Eurosurveillance 2011; 16. - PubMed

