Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies
- PMID: 22692007
- PMCID: PMC3518428
- DOI: 10.1016/j.gene.2012.06.004
Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies
Abstract
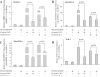
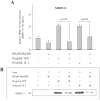
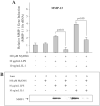
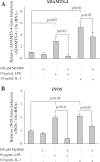
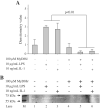
MyD88 is an adapter protein that links toll-like receptors (TLRs) and Interleukin-1 receptors (IL-1Rs) with downstream signaling molecules. The MyD88 has been found to be an essential mediator in the development of osteoarthritis in articular cartilage. However, the role of the MyD88 pathway has yet to be elucidated in the intervertebral disk (IVD). Using in vitro techniques, we analyzed the effect of MyD88 pathway-specific inhibition on the potent inflammatory and catabolic mediator LPS and IL-1 in bovine and human nucleus pulposus (NP) cells by assessing matrix-degrading enzyme expression, including matrix metalloproteases (MMPs) and a disintegrin-like and metalloprotease with thrombospondin motifs (ADAMTS family). We also analyzed inhibition of MyD88 in the regulation of inducible nitric oxide synthase and TLR-2. Finally, we used an ex vivo organ culture model to assess the effects of MyD88 inhibitor (MyD88i) on catabolic factor-induced disk degeneration in mice lumbar disks. In bovine NP cells, MyD88i potently antagonizes LPS- or IL-1-mediated induction of cartilage-degrading enzyme production, including MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5. MyD88i also attenuates the LPS- or IL-1-mediated induction of iNOS and TLR-2 gene expression. Our ex vivo findings reveal inhibition of MyD88 via counteraction of IL-1-mediated proteoglycan depletion. The findings from this study demonstrate the potent anti-inflammatory and anti-catabolic effects of inhibition of MyD88 pathway inhibition on IVD homeostasis, suggesting a potential therapeutic benefit of a MyD88i in degenerative disk disease in the future.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
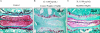
Similar articles
-
Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc.J Cell Physiol. 2013 Sep;228(9):1884-96. doi: 10.1002/jcp.24350. J Cell Physiol. 2013. PMID: 23460134 Free PMC article.
-
Crocin exerts anti-inflammatory and anti-catabolic effects on rat intervertebral discs by suppressing the activation of JNK.Int J Mol Med. 2015 Nov;36(5):1291-9. doi: 10.3892/ijmm.2015.2359. Epub 2015 Sep 30. Int J Mol Med. 2015. PMID: 26648423 Free PMC article.
-
Lactoferricin mediates anabolic and anti-catabolic effects in the intervertebral disc.J Cell Physiol. 2012 Apr;227(4):1512-20. doi: 10.1002/jcp.22867. J Cell Physiol. 2012. PMID: 21678402
-
Fibroblast growth factor control of cartilage homeostasis.J Cell Biochem. 2013 Apr;114(4):735-42. doi: 10.1002/jcb.24418. J Cell Biochem. 2013. PMID: 23060229 Free PMC article. Review.
-
Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration.Spine J. 2013 Mar;13(3):331-41. doi: 10.1016/j.spinee.2012.02.027. Epub 2013 Jan 29. Spine J. 2013. PMID: 23369495 Free PMC article. Review.
Cited by
-
Overexpression of TIMP3 inhibits discogenic pain by suppressing angiogenesis and the expression of substance P in nucleus pulposus.Mol Med Rep. 2020 Mar;21(3):1163-1171. doi: 10.3892/mmr.2020.10922. Epub 2020 Jan 9. Mol Med Rep. 2020. PMID: 31922222 Free PMC article.
-
MicroRNA-194 Inhibits Lipopolysaccharide-Induced Inflammatory Response in Nucleus Pulposus Cells of the Intervertebral Disc by Targeting TNF Receptor-Associated Factor 6 (TRAF6).Med Sci Monit. 2018 May 10;24:3056-3067. doi: 10.12659/MSM.907280. Med Sci Monit. 2018. PMID: 29745371 Free PMC article.
-
Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells.Eur Spine J. 2014 Sep;23(9):1878-91. doi: 10.1007/s00586-014-3442-4. Epub 2014 Jul 5. Eur Spine J. 2014. PMID: 24997157
-
Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration.Eur Cell Mater. 2015 Sep 21;30:104-16; discussion 116-7. doi: 10.22203/ecm.v030a08. Eur Cell Mater. 2015. PMID: 26388614 Free PMC article. Review.
-
Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis.J Cell Mol Med. 2020 Oct;24(20):11742-11754. doi: 10.1111/jcmm.15784. Epub 2020 Aug 29. J Cell Mol Med. 2020. PMID: 32860495 Free PMC article.
References
-
- Abdollahi-Roodsaz S, van de Loo FA, Koenders MI, Helsen MM, Walgreen B, van den Bersselaar LA, Arntz OJ, Takahashi N, Joosten LA, van den Berg WB. Destructive role of myeloid differentiation factor 88 and protective role of TIR-containing adaptor inducing interferon beta in IL-17-dependent arthritis. Arthritis Rheum. 2011;64:1838–1847. - PubMed
-
- Ahmad R, Sylvester J, Zafarullah M. MyD88, IRAK1 and TRAF6 knockdown in human chondrocytes inhibits interleukin-1-induced matrix metalloproteinase-13 gene expression and promoter activity by impairing MAP kinase activation. Cell. Signal. 2007;19:2549–2557. - PubMed
-
- An H, Boden SD, Kang J, Sandhu HS, Abdu W, Weinstein J. Summary statement: emerging techniques for treatment of degenerative lumbar disc disease. Spine (Phila Pa 1976) 2003a;28:S24–S25. - PubMed
-
- An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine (Phila Pa 1976) 2003b;28:S86–S92. - PubMed
-
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

