Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid
- PMID: 22692204
- PMCID: PMC3436511
- DOI: 10.1074/jbc.M112.364182
Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid
Abstract
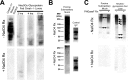
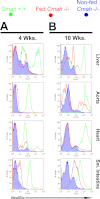
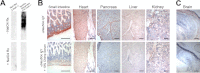
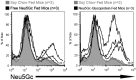
Although N-acetyl groups are common in nature, N-glycolyl groups are rare. Mammals express two major sialic acids, N-acetylneuraminic acid and N-glycolylneuraminic acid (Neu5Gc). Although humans cannot produce Neu5Gc, it is detected in the epithelial lining of hollow organs, endothelial lining of the vasculature, fetal tissues, and carcinomas. This unexpected expression is hypothesized to result via metabolic incorporation of Neu5Gc from mammalian foods. This accumulation has relevance for diseases associated with such nutrients, via interaction with Neu5Gc-specific antibodies. Little is known about how ingested sialic acids in general and Neu5Gc in particular are metabolized in the gastrointestinal tract. We studied the gastrointestinal and systemic fate of Neu5Gc-containing glycoproteins (Neu5Gc-glycoproteins) or free Neu5Gc in the Neu5Gc-free Cmah(-/-) mouse model. Ingested free Neu5Gc showed rapid absorption into the circulation and urinary excretion. In contrast, ingestion of Neu5Gc-glycoproteins led to Neu5Gc incorporation into the small intestinal wall, appearance in circulation at a steady-state level for several hours, and metabolic incorporation into multiple peripheral tissue glycoproteins and glycolipids, thus conclusively proving that Neu5Gc can be metabolically incorporated from food. Feeding Neu5Gc-glycoproteins but not free Neu5Gc mimics the human condition, causing tissue incorporation into human-like sites in Cmah(-/-) fetal and adult tissues, as well as developing tumors. Thus, glycoproteins containing glycosidically linked Neu5Gc are the likely dietary source for human tissue accumulation, and not the free monosaccharide. This human-like model can be used to elucidate specific mechanisms of Neu5Gc delivery from the gut to tissues, as well as general mechanisms of metabolism of ingested sialic acids.
Figures



References
-
- Schauer R. (1978) Characterization of sialic acids. Methods Enzymol. 50, 64–89 - PubMed
-
- Troy F. A. (1992) Polysialylation. From bacteria to brains. Glycobiology 2, 5–23 - PubMed
-
- Varki A., Schauer R. (2009) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds) pp. 199–218, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY - PubMed
-
- Shaw L., Schauer R. (1988) The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol. Chem. Hoppe-Seyler 369, 477–486 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

