IFN-γ-independent intraocular tumor rejection is mediated by a macrophage-dependent process that leaves the eye intact
- PMID: 22693246
- PMCID: PMC3476238
- DOI: 10.1189/jlb.0312122
IFN-γ-independent intraocular tumor rejection is mediated by a macrophage-dependent process that leaves the eye intact
Abstract
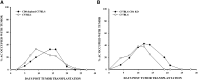
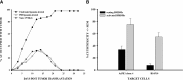
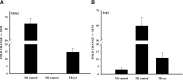
Intraocular tumors reside in an immune-privileged site, yet in certain circumstances, they can undergo immune rejection. Ocular tumor rejection can follow one of two pathways. One pathway is CD4(+) T cell-dependent and culminates in ischemic necrosis of the tumor and phthisis (atrophy) of the eye. A second pathway is also CD4(+) T cell-dependent but does not inflict collateral injury to ocular tissues, and the eye is preserved. We isolated two clones of a murine tumor, Ad5E1 that undergo profoundly different forms of immune rejection in the eye. Clone 2.1 tumors undergo an ischemic necrotizing form of rejection that requires IFN-γ, T cells, and ocular macrophages and culminates in destruction of the eye. By contrast, the second clone of Ad5E1, clone 4, undergoes rejection that also requires T cells and ocular macrophages, but leaves the eye in pristine condition (nonphthisical rejection). Here, we demonstrate that nonphthisical tumor rejection of clone 4 tumors is IFN-γ-independent but requires an ocular macrophage population that contains M1 and M2 macrophages. Clone 4 tumor-bearing eyes displayed ten- and 15-fold increases in M2- and M1-associated markers Arg1 and NO2, respectively. This is in sharp contrast to previous results with clone 2.1 tumor rejection, in which M2 markers were undetectable, and the eye was destroyed. These results suggest that the presence of M2 macrophages tempers the immune rejection of intraocular tumors and promotes immune effectors that inflict minimal injury to innocent bystander cells and thereby preserve the integrity and function of the eye.
Figures





Comment in
-
Editorial: A clear vision needs some balance.J Leukoc Biol. 2012 Nov;92(5):918-20. doi: 10.1189/jlb.0512247. J Leukoc Biol. 2012. PMID: 23118443 No abstract available.
References
-
- Whitsett C. F., Stulting R. D. (1984) The distribution of HLA antigens on human corneal tissue. Invest. Ophthalmol. Vis. Sci. 25, 519–524 - PubMed
-
- Streilein J. W. (2003) Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 3, 879–889 - PubMed
-
- Niederkorn J. Y. (2006) See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat. Immunol. 7, 354–359 - PubMed
-
- Niederkorn J. Y. (2002) Immune privilege in the anterior chamber of the eye. Crit. Rev. Immunol. 22, 13–46 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

